Exosomes derived from LPS-preconditioned bone marrow-derived MSC modulate macrophage plasticity to promote allograft survival via the NF-κB/NLRP3 signaling pathway
- PMID: 37716974
- PMCID: PMC10504750
- DOI: 10.1186/s12951-023-02087-8
Exosomes derived from LPS-preconditioned bone marrow-derived MSC modulate macrophage plasticity to promote allograft survival via the NF-κB/NLRP3 signaling pathway
Abstract
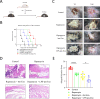
Objectives: This study investigated whether exosomes from LPS pretreated bone marrow mesenchymal stem cells (LPS pre-MSCs) could prolong skin graft survival.
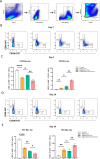
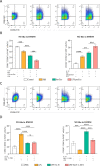
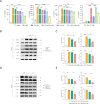
Methods: The exosomes were isolated from the supernatant of MSCs pretreated with LPS. LPS pre-Exo and rapamycin were injected via the tail vein into C57BL/6 mice allografted with BALB/c skin; graft survival was observed and evaluated. The accumulation and polarization of macrophages were examined by immunohistochemistry. The differentiation of macrophages in the spleen was analyzed by flow cytometry. For in vitro, an inflammatory model was established. Specifically, bone marrow-derived macrophages (BMDMs) were isolated and cultured with LPS (100 ng/ml) for 3 h, and were further treated with LPS pre-Exo for 24 h or 48 h. The molecular signaling pathway responsible for modulating inflammation was examined by Western blotting. The expressions of downstream inflammatory cytokines were determined by Elisa, and the polarization of macrophages was analyzed by flow cytometry.
Results: LPS pre-Exo could better ablate inflammation compared to untreated MSC-derived exosomes (BM-Exo). These loaded factors inhibited the expressions of inflammatory factors via a negative feedback mechanism. In vivo, LPS pre-Exo significantly attenuated inflammatory infiltration, thus improving the survival of allogeneic skin graft. Flow cytometric analysis of BMDMs showed that LPS pre-Exo were involved in the regulation of macrophage polarization and immune homeostasis during inflammation. Further investigation revealed that the NF-κB/NLRP3/procaspase-1/IL-1β signaling pathway played a key role in LPS pre-Exo-mediated regulation of macrophage polarization. Inhibiting NF-κB in BMDMs could abolish the LPS-induced activation of inflammatory pathways and the polarization of M1 macrophages while increasing the proportion of M2 cells.
Conclusion: LPS pre-Exo are able to switch the polarization of macrophages and enhance the resolution of inflammation. This type of exosomes provides an improved immunotherapeutic potential in prolonging graft survival.
Keywords: Allograft; Exosome; LPS preconditioning; Macrophage polarization; Mesenchymal stromal cells.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b.J Transl Med. 2015 Sep 19;13:308. doi: 10.1186/s12967-015-0642-6. J Transl Med. 2015. PMID: 26386558 Free PMC article.
-
Exosomes from LPS-preconditioned bone marrow MSCs accelerated peripheral nerve regeneration via M2 macrophage polarization: Involvement of TSG-6/NF-κB/NLRP3 signaling pathway.Exp Neurol. 2022 Oct;356:114139. doi: 10.1016/j.expneurol.2022.114139. Epub 2022 Jun 8. Exp Neurol. 2022. PMID: 35690131
-
Exosome-shuttled miR-150-5p from LPS-preconditioned mesenchymal stem cells down-regulate PI3K/Akt/mTOR pathway via Irs1 to enhance M2 macrophage polarization and confer protection against sepsis.Front Immunol. 2024 Jun 18;15:1397722. doi: 10.3389/fimmu.2024.1397722. eCollection 2024. Front Immunol. 2024. PMID: 38957471 Free PMC article.
-
Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization.J Cell Mol Med. 2019 Nov;23(11):7617-7631. doi: 10.1111/jcmm.14635. Epub 2019 Sep 26. J Cell Mol Med. 2019. PMID: 31557396 Free PMC article.
-
Exosome: Function and Application in Inflammatory Bone Diseases.Oxid Med Cell Longev. 2021 Aug 31;2021:6324912. doi: 10.1155/2021/6324912. eCollection 2021. Oxid Med Cell Longev. 2021. Retraction in: Oxid Med Cell Longev. 2024 Jan 9;2024:9806854. doi: 10.1155/2024/9806854. PMID: 34504641 Free PMC article. Retracted. Review.
Cited by
-
Targeting miR-29 mitigates skeletal senescence and bolsters therapeutic potential of mesenchymal stromal cells.Cell Rep Med. 2024 Aug 20;5(8):101665. doi: 10.1016/j.xcrm.2024.101665. Cell Rep Med. 2024. PMID: 39168101 Free PMC article.
-
Dexamethasone and IFN-γ primed mesenchymal stem cells conditioned media immunomodulates aberrant NETosis in SLE via PGE2 and IDO.Front Immunol. 2024 Oct 31;15:1461841. doi: 10.3389/fimmu.2024.1461841. eCollection 2024. Front Immunol. 2024. PMID: 39544931 Free PMC article.
-
The MSC-EV-microRNAome: A Perspective on Therapeutic Mechanisms of Action in Sepsis and ARDS.Cells. 2024 Jan 9;13(2):122. doi: 10.3390/cells13020122. Cells. 2024. PMID: 38247814 Free PMC article. Review.
-
Exosomal non-coding RNAs: Emerging insights into therapeutic potential and mechanisms in bone healing.J Tissue Eng. 2024 Oct 5;15:20417314241286606. doi: 10.1177/20417314241286606. eCollection 2024 Jan-Dec. J Tissue Eng. 2024. PMID: 39371940 Free PMC article. Review.
-
Optimizing therapeutic outcomes: preconditioning strategies for MSC-derived extracellular vesicles.Front Pharmacol. 2025 Feb 10;16:1509418. doi: 10.3389/fphar.2025.1509418. eCollection 2025. Front Pharmacol. 2025. PMID: 39995418 Free PMC article. Review.
References
-
- Prosser AC, Kallies A, Lucas M. Tissue-resident lymphocytes in solid organ transplantation: innocent passengers or the key to organ transplant survival? Transplantation. 2018;102(3):378–386. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

