LncRNA Neat1 targets NonO and miR-128-3p to promote antigen-specific Th17 cell responses and autoimmune inflammation
- PMID: 37716986
- PMCID: PMC10505237
- DOI: 10.1038/s41419-023-06132-0
LncRNA Neat1 targets NonO and miR-128-3p to promote antigen-specific Th17 cell responses and autoimmune inflammation
Abstract
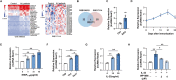
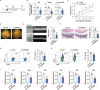
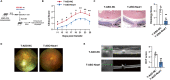
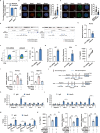
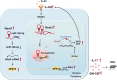
Long non-coding RNAs (lncRNAs) interaction with RNA-Binding proteins (RBPs) plays an important role in immunological processes. The generation of antigen-specific Th17 cells is closely associated with autoimmune pathogenesis. However, the function of lncRNA-RBP interactions in the regulation of pathogenic Th17 cell responses during autoimmunity remains poorly understood. Here, we found that lncRNA Neat1, highly expressed in Th17 cells, promoted antigen-specific Th17 cell responses. Both global and CD4+ T cell-specific knockdown of Neat1 protected mice against the development of experimental autoimmune uveitis (EAU). Mechanistically, Neat1 regulated RNA-Binding protein NonO, thus relieving IL-17 and IL-23R from NonO-mediated transcriptional repression and supporting antigen-specific Th17 cell responses. In addition, Neat1 also modulated miR-128-3p/NFAT5 axis to increase the expression of IL-17 and IL-23R, leading to augmented Th17 cell responses. Our findings elucidate a previously unrecognized mechanistic insight into the action of Neat1 in promoting antigen-specific Th17 responses and autoimmunity, and may facilitate the development of therapeutic targets for T cell-mediated autoimmune diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
miR-223-3p promotes autoreactive Th17 cell responses in experimental autoimmune uveitis (EAU) by inhibiting transcription factor FOXO3 expression.FASEB J. 2019 Dec;33(12):13951-13965. doi: 10.1096/fj.201901446R. Epub 2019 Oct 23. FASEB J. 2019. PMID: 31645142
-
Mettl3 induced miR-338-3p expression in dendritic cells promotes antigen-specific Th17 cell response via regulation of Dusp16.FASEB J. 2023 Nov;37(11):e23277. doi: 10.1096/fj.202300893R. FASEB J. 2023. PMID: 37878342
-
Knockdown of lncRNA NEAT1 inhibits Th17/CD4+ T cell differentiation through reducing the STAT3 protein level.J Cell Physiol. 2019 Dec;234(12):22477-22484. doi: 10.1002/jcp.28811. Epub 2019 May 22. J Cell Physiol. 2019. PMID: 31119756
-
LncRNAs involvement in pathogenesis of immune-related disease via regulation of T regulatory cells, an updated review.Cytokine. 2024 Jul;179:156585. doi: 10.1016/j.cyto.2024.156585. Epub 2024 Apr 4. Cytokine. 2024. PMID: 38579428 Review.
-
Molecular mechanism of lncRNAs in pathogenesis and diagnosis of auto-immune diseases, with a special focus on lncRNA-based therapeutic approaches.Life Sci. 2024 Jan 1;336:122322. doi: 10.1016/j.lfs.2023.122322. Epub 2023 Dec 1. Life Sci. 2024. PMID: 38042283 Review.
Cited by
-
LINC01089 in cancer: multifunctional roles and therapeutic implications.J Transl Med. 2024 Sep 27;22(1):858. doi: 10.1186/s12967-024-05693-8. J Transl Med. 2024. PMID: 39334363 Free PMC article. Review.
-
Emerging Role of Long, Non-Coding RNA Nuclear-Enriched Abundant Transcript 1 in Stress- and Immune-Related Diseases.Int J Mol Sci. 2025 May 6;26(9):4413. doi: 10.3390/ijms26094413. Int J Mol Sci. 2025. PMID: 40362651 Free PMC article. Review.
-
Differential Protein Expression in Extracellular Vesicles Defines Treatment Responders and Non-Responders in Multiple Sclerosis.Int J Mol Sci. 2024 Oct 6;25(19):10761. doi: 10.3390/ijms251910761. Int J Mol Sci. 2024. PMID: 39409091 Free PMC article.
-
Defining the transcriptional routes controlling lncRNA NEAT1 expression: implications in cellular stress response, inflammation, and differentiation.Discov Oncol. 2025 May 15;16(1):768. doi: 10.1007/s12672-025-02510-6. Discov Oncol. 2025. PMID: 40369379 Free PMC article. Review.
-
Fetal hypoplastic lungs have multilineage inflammation that is reversed by amniotic fluid stem cell extracellular vesicle treatment.Sci Adv. 2024 Jul 26;10(30):eadn5405. doi: 10.1126/sciadv.adn5405. Epub 2024 Jul 26. Sci Adv. 2024. PMID: 39058789 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

