Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification
- PMID: 37717008
- PMCID: PMC10505332
- DOI: 10.1186/s12951-023-02081-0
Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification
Abstract
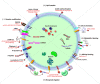
Extracellular vesicles (EVs) are 30-150 nm membrane-bound vesicles naturally secreted by cells and play important roles in intercellular communication by delivering regulatory molecules such as proteins, lipids, nucleic acids and metabolites to recipient cells. As natural nano-carriers, EVs possess desirable properties such as high biocompatibility, biological barrier permeability, low toxicity, and low immunogenicity, making them potential therapeutic delivery vehicles. EVs derived from specific cells have inherent targeting capacity towards specific cell types, which is yet not satisfactory enough for targeted therapy development and needs to be improved. Surface modifications endow EVs with targeting abilities, significantly improving their therapeutic efficiency. Herein, we first briefly introduce the biogenesis, composition, uptake and function of EVs, and review the cargo loading approaches for EVs. Then, we summarize the recent advances in surface engineering strategies of EVs, focusing on the applications of engineered EVs for targeted therapy. Altogether, EVs hold great promise for targeted delivery of various cargos, and targeted modifications show promising effects on multiple diseases.
Keywords: Exosome; Extracellular vesicles; Surface modification; Targeted delivery.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



Similar articles
-
Achieving the Promise of Therapeutic Extracellular Vesicles: The Devil is in Details of Therapeutic Loading.Pharm Res. 2017 May;34(5):1053-1066. doi: 10.1007/s11095-017-2123-5. Epub 2017 Mar 17. Pharm Res. 2017. PMID: 28315083 Free PMC article. Review.
-
Membrane Derived Vesicles as Biomimetic Carriers for Targeted Drug Delivery System.Curr Top Med Chem. 2020;20(27):2472-2492. doi: 10.2174/1568026620666200922113054. Curr Top Med Chem. 2020. PMID: 32962615 Review.
-
Extracellular vesicles (EVs)' journey in recipient cells: from recognition to cargo release.J Zhejiang Univ Sci B. 2024 Aug 15;25(8):633-655. doi: 10.1631/jzus.B2300566. J Zhejiang Univ Sci B. 2024. PMID: 39155778 Free PMC article. Review.
-
Extracellular vesicles: a rising star for therapeutics and drug delivery.J Nanobiotechnology. 2023 Jul 20;21(1):231. doi: 10.1186/s12951-023-01973-5. J Nanobiotechnology. 2023. PMID: 37475025 Free PMC article. Review.
-
Recent Progress of Extracellular Vesicle Engineering.ACS Biomater Sci Eng. 2021 Sep 13;7(9):4430-4438. doi: 10.1021/acsbiomaterials.1c00868. Epub 2021 Aug 28. ACS Biomater Sci Eng. 2021. PMID: 34455789
Cited by
-
Extracellular Vesicles in Idiopathic Pulmonary Fibrosis: Pathogenesis, Biomarkers and Innovative Therapeutic Strategies.Int J Nanomedicine. 2024 Nov 25;19:12593-12614. doi: 10.2147/IJN.S491335. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39619058 Free PMC article. Review.
-
Harnessing Extracellular Vesicles for Targeted Drug Delivery in Ovarian Cancer.Pharmaceutics. 2025 Apr 17;17(4):528. doi: 10.3390/pharmaceutics17040528. Pharmaceutics. 2025. PMID: 40284522 Free PMC article. Review.
-
Engineered extracellular vesicles with sequential cell recruitment and osteogenic functions to effectively promote senescent bone repair.J Nanobiotechnology. 2025 Feb 12;23(1):107. doi: 10.1186/s12951-025-03168-6. J Nanobiotechnology. 2025. PMID: 39939879 Free PMC article.
-
Neural Stem Cell-Derived Extracellular Vesicles for Advanced Neural Repair.J Neurochem. 2025 Aug;169(8):e70170. doi: 10.1111/jnc.70170. J Neurochem. 2025. PMID: 40734485 Free PMC article. Review.
-
Basic Guide for Approaching Drug Delivery with Extracellular Vesicles.Int J Mol Sci. 2024 Sep 27;25(19):10401. doi: 10.3390/ijms251910401. Int J Mol Sci. 2024. PMID: 39408730 Free PMC article. Review.
References
-
- Johnstone RM. The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem Cell Biol Biochim et Biol Cell. 1992;70(3–4):179–190. - PubMed
-
- Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44(11):2060–2064. - PubMed
-
- Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, et al. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13(9):1738–1755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

