Analgesic efficacy of collagen peptide in knee osteoarthritis: a meta-analysis of randomized controlled trials
- PMID: 37717022
- PMCID: PMC10505327
- DOI: 10.1186/s13018-023-04182-w
Analgesic efficacy of collagen peptide in knee osteoarthritis: a meta-analysis of randomized controlled trials
Abstract
Background: The management of knee osteoarthritis involves various treatment strategies. It is important to explore alternative therapies that are both safe and effective. Collagen peptides have emerged as a potential intervention for knee osteoarthritis. This study aims to evaluate the analgesic effects and safety of collagen peptide in patients diagnosed with knee osteoarthritis.
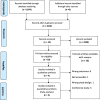
Methods: We conducted a systematic literature search following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Multiple databases including PubMed, Scopus, EMBASE, Web of Science, Cochrane, and ClinicalTrials.gov were searched for randomized controlled trials (RCTs) published up to 27 May 2023 that focused on the analgesic outcomes and adverse events associated with collagen peptides or hydrolyzed collagen in patients with osteoarthritis. We assessed the quality of the included studies and the strength of evidence using the Cochrane ROB 2.0 tool and Grading of Recommendations, Assessment, Development, and Evaluations.
Results: Four trials involving 507 patients with knee osteoarthritis were included and analyzed using the random-effects model. All these trials were considered to have a high risk of bias. Our results revealed a significant difference in pain relief between the collagen peptide group and the placebo group in patients with knee osteoarthritis (standardized mean difference: - 0.58; 95% CI - 0.98, - 0.18, p = 0.004; I2: 68%; quality of evidence: moderate). However, there was no significant difference in the risk of adverse events between collagen peptide and placebo (odds ratio: 1.66; 95% CI 0.99, 2.78, p = 0.05; I2: 0%; quality of evidence: very low).
Conclusions: Our findings demonstrate significant pain relief in patients with knee osteoarthritis who received collagen peptides compared to those who received placebo. In addition, the risk of adverse events did not differ significantly between the collagen peptide group and the placebo group. However, due to potential biases and limitations, well-designed randomized controlled trials are needed to validate and confirm these findings.
Keywords: Adverse events; Collagen peptide; Knee osteoarthritis; Osteoarthritis; Pain.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
Financial, commercial, or other relationships were absent in this research. There was no conflict of interest in this research.
Figures





Similar articles
-
Intra-articular viscosupplementation with hylan g-f 20 to treat osteoarthritis of the knee: an evidence-based analysis.Ont Health Technol Assess Ser. 2005;5(10):1-66. Epub 2005 Jun 1. Ont Health Technol Assess Ser. 2005. PMID: 23074461 Free PMC article.
-
Tramadol for osteoarthritis.Cochrane Database Syst Rev. 2019 May 27;5(5):CD005522. doi: 10.1002/14651858.CD005522.pub3. Cochrane Database Syst Rev. 2019. PMID: 31132298 Free PMC article.
-
Efficacy and Safety of Duloxetine on Osteoarthritis Knee Pain: A Meta-Analysis of Randomized Controlled Trials.Pain Med. 2015 Jul;16(7):1373-85. doi: 10.1111/pme.12800. Epub 2015 Jun 5. Pain Med. 2015. PMID: 26176791
-
Efficacy and Safety of Tanezumab on Osteoarthritis Knee and Hip Pains: A Meta-Analysis of Randomized Controlled Trials.Pain Med. 2017 Feb 1;18(2):374-385. doi: 10.1093/pm/pnw262. Pain Med. 2017. PMID: 28034979
-
Effects of Whole-Body Vibration Therapy on Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.J Rehabil Med. 2022 Mar 29;54:jrm00266. doi: 10.2340/jrm.v54.2032. J Rehabil Med. 2022. PMID: 35174868 Free PMC article.
Cited by
-
Local and Systemic Peptide Therapies for Soft Tissue Regeneration: A Narrative Review.Yale J Biol Med. 2024 Sep 30;97(3):399-413. doi: 10.59249/TKNM3388. eCollection 2024 Sep. Yale J Biol Med. 2024. PMID: 39351323 Free PMC article. Review.
-
Oral administration of hydrolyzed collagen alleviates pain and enhances functionality in knee osteoarthritis: Results from a randomized, double-blind, placebo-controlled study.Contemp Clin Trials Commun. 2024 Dec 30;43:101424. doi: 10.1016/j.conctc.2024.101424. eCollection 2025 Feb. Contemp Clin Trials Commun. 2024. PMID: 39839727 Free PMC article.
-
Effect of supplementation with type 1 and type 3 collagen peptide and type 2 hydrolyzed collagen on osteoarthritis-related pain, quality of life, and physical function: A double-blind, randomized, placebo-controlled study.Jt Dis Relat Surg. 2025 Jan 2;36(1):85-96. doi: 10.52312/jdrs.2025.1965. Epub 2024 Nov 5. Jt Dis Relat Surg. 2025. PMID: 39719905 Free PMC article. Clinical Trial.
References
-
- Hsu H, Siwiec RM. Knee osteoarthritis. Treasure Island (FL): StatPearls Publishing; 2022. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

