Mild sleep restriction increases endothelial oxidative stress in female persons
- PMID: 37717072
- PMCID: PMC10505226
- DOI: 10.1038/s41598-023-42758-y
Mild sleep restriction increases endothelial oxidative stress in female persons
Abstract
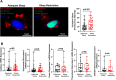
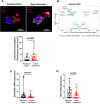
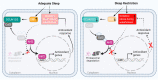
Sleep restriction is associated with increased cardiovascular risk, which is more pronounced in female than male persons. We reported recently first causal evidence that mild, prolonged sleep restriction mimicking "real-life" conditions impairs endothelial function, a key step in the development and progression of cardiovascular disease, in healthy female persons. However, the underlying mechanisms are unclear. In model organisms, sleep restriction increases oxidative stress and upregulates antioxidant response via induction of the antioxidant regulator nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Here, we assessed directly endothelial cell oxidative stress and antioxidant responses in healthy female persons (n = 35) after 6 weeks of mild sleep restriction (1.5 h less than habitual sleep) using randomized crossover design. Sleep restriction markedly increased endothelial oxidative stress without upregulating antioxidant response. Using RNA-seq and a predicted protein-protein interaction database, we identified reduced expression of endothelial Defective in Cullin Neddylation-1 Domain Containing 3 (DCUN1D3), a protein that licenses Nrf2 antioxidant responses, as a mediator of impaired endothelial antioxidant response in sleep restriction. Thus, sleep restriction impairs clearance of endothelial oxidative stress that over time increases cardiovascular risk.Trial Registration: NCT02835261 .
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

