Proposal of recommended experimental protocols for in vitro and in vivo evaluation methods of boron agents for neutron capture therapy
- PMID: 37717596
- PMCID: PMC10665309
- DOI: 10.1093/jrr/rrad064
Proposal of recommended experimental protocols for in vitro and in vivo evaluation methods of boron agents for neutron capture therapy
Erratum in
-
Correction to: Proposal of recommended experimental protocols for in vitro and in vivo evaluation methods of boron agents for neutron capture therapy.J Radiat Res. 2023 Nov 21;64(6):983. doi: 10.1093/jrr/rrad078. J Radiat Res. 2023. PMID: 37775502 Free PMC article. No abstract available.
Abstract
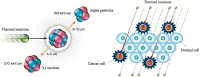
Recently, boron neutron capture therapy (BNCT) has been attracting attention as a minimally invasive cancer treatment. In 2020, the accelerator-based BNCT with L-BPA (Borofalan) as its D-sorbitol complex (Steboronine®) for head and neck cancers was approved by Pharmaceutical and Medical Devices Agency for the first time in the world. As accelerator-based neutron generation techniques are being developed in various countries, the development of novel tumor-selective boron agents is becoming increasingly important and desired. The Japanese Society of Neutron Capture Therapy believes it is necessary to propose standard evaluation protocols at each stage in the development of boron agents for BNCT. This review summarizes recommended experimental protocols for in vitro and in vivo evaluation methods of boron agents for BNCT based on our experience with L-BPA approval.
Keywords: in vitro; in vivo; boron agents; boron neutron capture therapy (BNCT); evaluation protocols.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Japanese Radiation Research Society and Japanese Society for Radiation Oncology.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Barth RF. Boron neutron capture therapy at the crossroads: challenges and opportunities. Appl Radiat Isot 2009;67:S3–6. - PubMed
-
- Moss RL. Critical review, with an optimistic outlook, on boron neutron capture therapy (BNCT). Appl Radiat Isot 2014;88:2–11. - PubMed
-
- Suzuki M, Tanaka H, Sakurai Y et al. Impact of accelerator-based boron neutron capture therapy (AB-BNCT) on the treatment of multiple liver tumors and malignant pleural mesothelioma. Radiother Oncol 2009;92:89–95. - PubMed
-
- Tanaka H, Sakurai Y, Suzuki M et al. Experimental verification of beam characteristics for cyclotron-based epithermal neutron source (C-BENS). Appl Radiat Isot 2011;69:1642–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

