Development and testing of a metabolic chamber for effluent collection during whole eye perfusions
- PMID: 37717688
- PMCID: PMC10842592
- DOI: 10.1016/j.exer.2023.109652
Development and testing of a metabolic chamber for effluent collection during whole eye perfusions
Abstract
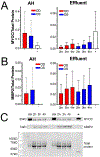
Ocular hypertension is caused by dysregulated outflow resistance regulation by the conventional outflow (CO) pathway. The physiology of the CO pathway can be directly studied during ex vivo ocular perfusions. In addition to measuring outflow resistance generation by the CO tissues, perfusion media that is conditioned by CO pathway cells can be collected upon exiting the eye as effluent. Thus, contents of effluent include factors contributed by upstream cells that report on the (dys)functionality of the outflow tissues. Two methods have been used in the past to monitor effluent contents from perfused eyes, each with their limitations. To overcome these limitations, we designed and printed a metabolic chamber to accommodate eyes of different sizes during perfusions. To test this new chamber, human eyes were perfused for 4 h at constant flow rate of 2.5 μl/min, while pressure was continuously monitored and effluent was collected every hour. Facility was 0.28 ± 0.16 μl/min/mmHg for OD eyes and 0.33 ± 0.11 μl/min/mmHg for OS eyes (n = 3). Effluent samples were protein rich, with protein concentration ranging from 2700 to 10,000 μg/ml for all eyes and timepoints (N = 3). Effluent samples expressed proteins that were actively secreted by the TM and easily detectible including MYOC and MMP2. Taken together, our model provides a reliable method to collect effluent from ex vivo human eyes, while maintaining whole globe integrity.
Keywords: Aqueous humor dynamics; Conventional; Effluent; Glaucoma; Ocular perfusion.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interest:
None
Figures




Similar articles
-
Anterior chamber perfusion versus posterior chamber perfusion does not influence measurement of aqueous outflow facility in living mice by constant flow infusion.Exp Eye Res. 2017 Nov;164:95-108. doi: 10.1016/j.exer.2017.08.011. Epub 2017 Aug 16. Exp Eye Res. 2017. PMID: 28822760
-
Greater Outflow Facility Increase After Targeted Trabecular Bypass in Angiographically Determined Low-low Regions.Ophthalmol Glaucoma. 2023 Nov-Dec;6(6):570-579. doi: 10.1016/j.ogla.2023.06.008. Epub 2023 Jun 20. Ophthalmol Glaucoma. 2023. PMID: 37348815 Free PMC article.
-
Morphological changes to Schlemm's canal and the distal aqueous outflow pathway in monkey eyes with laser-induced ocular hypertension.Exp Eye Res. 2022 Jun;219:109030. doi: 10.1016/j.exer.2022.109030. Epub 2022 Mar 10. Exp Eye Res. 2022. PMID: 35283108 Free PMC article.
-
Pressure-induced expression changes in segmental flow regions of the human trabecular meshwork.Exp Eye Res. 2017 May;158:67-72. doi: 10.1016/j.exer.2016.06.009. Epub 2016 Jun 19. Exp Eye Res. 2017. PMID: 27334250 Free PMC article. Review.
-
Deconstructing aqueous humor outflow - The last 50 years.Exp Eye Res. 2020 Aug;197:108105. doi: 10.1016/j.exer.2020.108105. Epub 2020 Jun 23. Exp Eye Res. 2020. PMID: 32590004 Free PMC article. Review.
Cited by
-
ANGPTL7 and Its Role in IOP and Glaucoma.Invest Ophthalmol Vis Sci. 2024 Mar 5;65(3):22. doi: 10.1167/iovs.65.3.22. Invest Ophthalmol Vis Sci. 2024. PMID: 38497513 Free PMC article.
References
-
- Adam MF, Belmouden A, Binisti P, Brézin AP, Valtot F, Béchetoille A, Dascotte J-C, Copin B, Gomez L and Chaventré A (1997). “Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma.” Human molecular genetics 6(12): 2091–2097. - PubMed
-
- Alexander JP, Samples JR, Van Buskirk EM and Acott TS (1991). “Expression of matrix metalloproteinases and inhibitor by human trabecular meshwork.” Investigative ophthalmology & visual science 32(1): 172–180. - PubMed
-
- Allingham RR, de Kater AW, Ethier CR, Anderson PJ, Hertzmark E and Epstein DL (1992). “The relationship between pore density and outflow facility in human eyes.” Investigative ophthalmology & visual science 33(5): 1661–1669. - PubMed
-
- Bahler CK, Fautsch MP, Hann CR and Johnson DH (2004). “Factors influencing intraocular pressure in cultured human anterior segments.” Investigative ophthalmology & visual science 45(9): 3137–3143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

