Adolescent Alcohol Exposure Produces Sex-Specific Long-term Hyperalgesia via Changes in Central Amygdala Circuit Function
- PMID: 37717844
- PMCID: PMC10866691
- DOI: 10.1016/j.biopsych.2023.09.006
Adolescent Alcohol Exposure Produces Sex-Specific Long-term Hyperalgesia via Changes in Central Amygdala Circuit Function
Abstract
Background: Exposure to alcohol during adolescence produces many effects that last well into adulthood. Acute alcohol use is analgesic, and people living with pain report drinking alcohol to reduce pain, but chronic alcohol use produces increases in pain sensitivity.
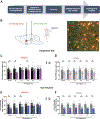
Methods: We tested the acute and lasting effects of chronic adolescent intermittent ethanol (AIE) exposure on pain-related behavioral and brain changes in male and female rats. We also tested the long-term effects of AIE on synaptic transmission in midbrain (ventrolateral periaqueductal gray [vlPAG])-projecting central amygdala (CeA) neurons using whole-cell electrophysiology. Finally, we used circuit-based approaches (DREADDs [designer receptors exclusively activated by designer drugs]) to test the role of vlPAG-projecting CeA neurons in mediating AIE effects on pain-related outcomes.
Results: AIE produced long-lasting hyperalgesia in male, but not female, rats. Similarly, AIE led to a reduction in synaptic strength of medial CeA cells that project to the vlPAG in male, but not female, rats. Challenge with an acute painful stimulus (i.e., formalin) in adulthood produced expected increases in pain reactivity, and this effect was exaggerated in male rats with a history of AIE. Finally, CeA-vlPAG circuit activation rescued AIE-induced hypersensitivity in male rats.
Conclusions: Our findings are the first, to our knowledge, to show long-lasting sex-dependent effects of adolescent alcohol exposure on pain-related behaviors and brain circuits in adult animals. This work has implications for understanding the long-term effects of underage alcohol drinking on pain-related behaviors in humans.
Keywords: Adolescents; Alcohol; Central amygdala; Pain; Rats; Ventral lateral periaqueductal gray.
Copyright © 2023 Society of Biological Psychiatry. All rights reserved.
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest.
Figures





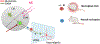
References
-
- Chou SP and Pickering RP (1992): Early onset of drinking as a risk factor for lifetime alcohol-related problems. British Journal of Addiction 87: 1199–1204. - PubMed
-
- Spear LP (2000): Neurobehavioral Changes in Adolescence. Current Directions in Psychological Science 9(4), 111–114.
-
- Substance Abuse and Mental Health Services Administration (2021): Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21–07-01–003, NSDUH Series H-56). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration.
-
- Hoftun GB, Romundstad PR, Rygg M (2012): Factors associated with adolescent chronic non-specific pain, chronic multisite pain, and chronic pain with high disability: the Young-HUNT Study 2008. J Pain Sep;13(9):874–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

