Genomic Staging of Multifocal Lung Squamous Cell Carcinomas Is Independent of the Comprehensive Morphologic Assessment
- PMID: 37717856
- PMCID: PMC11866686
- DOI: 10.1016/j.jtho.2023.09.275
Genomic Staging of Multifocal Lung Squamous Cell Carcinomas Is Independent of the Comprehensive Morphologic Assessment
Abstract
Introduction: Morphologic and molecular data for staging of multifocal lung squamous cell carcinomas (LSCCs) are limited. In this study, whole exome sequencing (WES) was used as the gold standard to determine whether multifocal LSCC represented separate primary lung cancers (SPLCs) or intrapulmonary metastases (IPMs). Genomic profiles were compared with the comprehensive morphologic assessment.
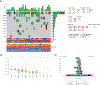
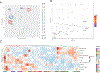
Methods: WES was performed on 20 tumor pairs of multifocal LSCC and matched normal lymph nodes using the Illumina NovaSeq6000 S4-Xp (Illumina, San Diego, CA). WES clonal and subclonal analysis data were compared with histologic assessment by 16 thoracic pathologists. In addition, the immune gene profiling of the study cases was characterized by the HTG EdgeSeq Precision Immuno-Oncology Panel.
Results: By WES data, 11 cases were classified as SPLC and seven cases as IPM. Two cases were technically suboptimal. Analysis revealed marked genomic and immunogenic heterogeneity, but immune gene expression profiles highly correlated with mutation profiles. Tumors classified as IPM have a large number of shared mutations (ranging from 33.5% to 80.7%). The agreement between individual morphologic assessments for each case and WES was 58.3%. One case was unanimously interpreted morphologically as IPM and was in agreement with WES. In a further 17 cases, the number of pathologists whose morphologic interpretation was in agreement with WES ranged from two (one case) to 15 pathologists (one case) per case. Pathologists showed a fair interobserver agreement in the morphologic staging of multiple LSCCs, with an overall kappa of 0.232.
Conclusions: Staging of multifocal LSCC based on morphologic assessment is unreliable. Comprehensive genomic analyses should be adopted for the staging of multifocal LSCC.
Keywords: Histologic subtyping; Multifocal lung cancer; Squamous; Staging; WES.
Copyright © 2023 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The remaining authors declare no conflict of interest.
Figures



References
-
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70(4):606–612. - PubMed
-
- Detterbeck FC, Franklin WA, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: Background Data and Proposed Criteria to Distinguish Separate Primary Lung Cancers from Metastatic Foci in Patients with Two Lung Tumors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11(5):651–665. - PubMed
-
- Travis WD BE, Burke AP, Marx A, Nicholson AG. WHO Classification of tumors of the lung, pleura, thymus and heart. Vol 7. Lyon: IARC Press; 2015. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

