Estrogen-Related Receptor Agonism Reverses Mitochondrial Dysfunction and Inflammation in the Aging Kidney
- PMID: 37717940
- PMCID: PMC10734281
- DOI: 10.1016/j.ajpath.2023.07.008
Estrogen-Related Receptor Agonism Reverses Mitochondrial Dysfunction and Inflammation in the Aging Kidney
Abstract
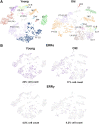
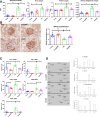
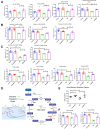
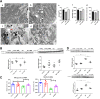
A gradual decline in renal function occurs even in healthy aging individuals. In addition to aging, per se, concurrent metabolic syndrome and hypertension, which are common in the aging population, can induce mitochondrial dysfunction and inflammation, which collectively contribute to age-related kidney dysfunction and disease. This study examined the role of the nuclear hormone receptors, the estrogen-related receptors (ERRs), in regulation of age-related mitochondrial dysfunction and inflammation. The ERRs were decreased in both aging human and mouse kidneys and were preserved in aging mice with lifelong caloric restriction (CR). A pan-ERR agonist, SLU-PP-332, was used to treat 21-month-old mice for 8 weeks. In addition, 21-month-old mice were treated with a stimulator of interferon genes (STING) inhibitor, C-176, for 3 weeks. Remarkably, similar to CR, an 8-week treatment with a pan-ERR agonist reversed the age-related increases in albuminuria, podocyte loss, mitochondrial dysfunction, and inflammatory cytokines, via the cyclic GMP-AMP synthase-STING and STAT3 signaling pathways. A 3-week treatment of 21-month-old mice with a STING inhibitor reversed the increases in inflammatory cytokines and the senescence marker, p21/cyclin dependent kinase inhibitor 1A (Cdkn1a), but also unexpectedly reversed the age-related decreases in PPARG coactivator (PGC)-1α, ERRα, mitochondrial complexes, and medium chain acyl coenzyme A dehydrogenase (MCAD) expression. These studies identified ERRs as CR mimetics and as important modulators of age-related mitochondrial dysfunction and inflammation. These findings highlight novel druggable pathways that can be further evaluated to prevent progression of age-related kidney disease.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure Statement None declared.
Figures



References
-
- Tonelli M., Riella M. Chronic kidney disease and the aging population. Am J Physiol Ren Physiol. 2014;306:F469–F472. - PubMed
-
- Choudhury D., Levi M. Kidney aging--inevitable or preventable? Nat Rev Nephrol. 2011;7:706–717. - PubMed
-
- Berg U.B. Differences in decline in GFR with age between males and females: reference data on clearances of inulin and PAH in potential kidney donors. Nephrol Dial Transplant. 2006;21:2577–2582. - PubMed
-
- Tauchi H., Tsuboi K., Okutomi J. Age changes in the human kidney of the different races. Gerontologia. 1971;17:87–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

