Resolvin D2-G-Protein Coupled Receptor 18 Enhances Bone Marrow Function and Limits Steatosis and Hepatic Collagen Accumulation in Aging
- PMID: 37717941
- PMCID: PMC10699127
- DOI: 10.1016/j.ajpath.2023.08.011
Resolvin D2-G-Protein Coupled Receptor 18 Enhances Bone Marrow Function and Limits Steatosis and Hepatic Collagen Accumulation in Aging
Abstract
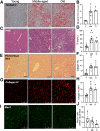
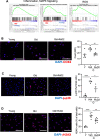
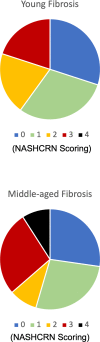
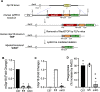
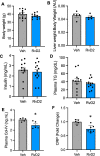
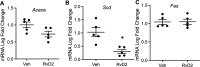
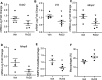
Aging is associated with nonresolving inflammation and tissue dysfunction. Resolvin D2 (RvD2) is a proresolving ligand that acts through the G-protein-coupled receptor called GPR18. Unbiased RNA sequencing revealed increased Gpr18 expression in macrophages from old mice, and in livers from elderly humans, which was associated with increased steatosis and fibrosis in middle-aged (MA) and old mice. MA mice that lacked GPR18 on myeloid cells had exacerbated steatosis and hepatic fibrosis, which was associated with a decline in Mac2+ macrophages. Treatment of MA mice with RvD2 reduced steatosis and decreased hepatic fibrosis, correlating with increased Mac2+ macrophages, increased monocyte-derived macrophages, and elevated numbers of monocytes in the liver, blood, and bone marrow. RvD2 acted directly on the bone marrow to increase monocyte-macrophage progenitors. A transplantation assay further demonstrated that bone marrow from old mice facilitated hepatic collagen accumulation in young mice. Transient RvD2 treatment to mice transplanted with bone marrow from old mice prevented hepatic collagen accumulation. Together, this study demonstrates that RvD2-GPR18 signaling controls steatosis and fibrosis and provides a mechanistic-based therapy for promoting liver repair in aging.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures









Update of
-
The Resolvin D2-GPR18 Axis Enhances Bone Marrow Function and Limits Hepatic Fibrosis in Aging.bioRxiv [Preprint]. 2023 Jan 6:2023.01.05.522881. doi: 10.1101/2023.01.05.522881. bioRxiv. 2023. Update in: Am J Pathol. 2023 Dec;193(12):1953-1968. doi: 10.1016/j.ajpath.2023.08.011. PMID: 36711905 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

