Regulating Blood Clot Fibrin Films to Manipulate Biomaterial-Mediated Foreign Body Responses
- PMID: 37719049
- PMCID: PMC10503960
- DOI: 10.34133/research.0225
Regulating Blood Clot Fibrin Films to Manipulate Biomaterial-Mediated Foreign Body Responses
Abstract
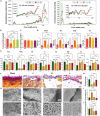
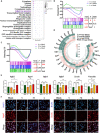
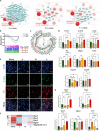
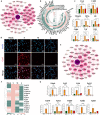
The clinical efficacy of implanted biomaterials is often compromised by host immune recognition and subsequent foreign body responses (FBRs). During the implantation, biomaterials inevitably come into direct contact with the blood, absorbing blood protein and forming blood clot. Many studies have been carried out to regulate protein adsorption, thus manipulating FBR. However, the role of clot surface fibrin films formed by clotting shrinkage in host reactions and FBR is often ignored. Because of the principle of fibrin film formation being relevant to fibrinogen or clotting factor absorption, it is feasible to manipulate the fibrin film formation via tuning the absorption of fibrinogen and clotting factor. As biological hydroxyapatite reserved bone architecture and microporous structure, the smaller particle size may expose more microporous structures and adsorb more fibrinogen or clotting factor. Therefore, we set up 3 sizes (small, <0.2 mm; medium, 1 to 2 mm; large, 3 to 4 mm) of biological hydroxyapatite (porcine bone-derived hydroxyapatite) with different microporous structures to investigate the absorption of blood protein, the formation of clot surface fibrin films, and the subsequent FBR. We found that small group adsorbed more clotting factors because of more microporous structures and formed the thinnest and sparsest fibrin films. These thinnest and sparsest fibrin films increased inflammation and profibrosis of macrophages through a potential signaling pathway of cell adhesion-cytoskeleton-autophagy, leading to the stronger FBR. Large group adsorbed lesser clotting factors, forming the thickest and densest fibrin films, easing inflammation and profibrosis of macrophages, and finally mitigating FBR. Thus, this study deepens the understanding of the role of fibrin films in host recognition and FBR and demonstrates the feasibility of a strategy to regulate FBR by modulating fibrin films via tuning the absorption of blood proteins.
Copyright © 2023 Yang Zou et al.
Figures


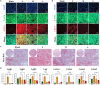
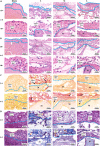
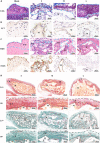

Similar articles
-
Fibrin polymer on the surface of biomaterial implants drives the foreign body reaction.Biomaterials. 2021 Oct;277:121087. doi: 10.1016/j.biomaterials.2021.121087. Epub 2021 Aug 25. Biomaterials. 2021. PMID: 34478933 Free PMC article.
-
Mesopore Controls the Responses of Blood Clot-Immune Complex via Modulating Fibrin Network.Adv Sci (Weinh). 2022 Jan;9(3):e2103608. doi: 10.1002/advs.202103608. Epub 2021 Nov 24. Adv Sci (Weinh). 2022. PMID: 34821070 Free PMC article.
-
An in vitro model that mimics the foreign body response in the peritoneum: Study of the bioadhesive properties of HA-based materials.Carbohydr Polym. 2023 Jun 15;310:120701. doi: 10.1016/j.carbpol.2023.120701. Epub 2023 Feb 17. Carbohydr Polym. 2023. PMID: 36925239
-
Decoding biomaterial-associated molecular patterns (BAMPs): influential players in bone graft-related foreign body reactions.PeerJ. 2025 Apr 22;13:e19299. doi: 10.7717/peerj.19299. eCollection 2025. PeerJ. 2025. PMID: 40292103 Free PMC article. Review.
-
The pathology of the foreign body reaction against biomaterials.J Biomed Mater Res A. 2017 Mar;105(3):927-940. doi: 10.1002/jbm.a.35958. Epub 2016 Nov 25. J Biomed Mater Res A. 2017. PMID: 27813288 Review.
Cited by
-
Anti-Scar Effects of Micropatterned Hydrogel after Glaucoma Drainage Device Implantation.Research (Wash D C). 2025 Jan 22;8:0561. doi: 10.34133/research.0561. eCollection 2025. Research (Wash D C). 2025. PMID: 39845708 Free PMC article.
-
Biomimetic peptide conjugates as emerging strategies for controlled release from protein-based materials.Drug Deliv. 2025 Dec;32(1):2449703. doi: 10.1080/10717544.2025.2449703. Epub 2025 Jan 9. Drug Deliv. 2025. PMID: 39782014 Free PMC article. Review.
-
The paradigm shifts of periodontal regeneration strategy: From reparative manipulation to developmental engineering.Bioact Mater. 2025 Mar 18;49:418-436. doi: 10.1016/j.bioactmat.2025.03.009. eCollection 2025 Jul. Bioact Mater. 2025. PMID: 40165829 Free PMC article. Review.
-
Evolution of Biological Hydroxyapatite Modification Strategy: Anti-Inflammation Approach Rescues the Calcium-NOD-Like Receptor-Inflammation Axis-Mediated Periodontal Redevelopment Failure.Biomater Res. 2025 Feb 26;29:0131. doi: 10.34133/bmr.0131. eCollection 2025. Biomater Res. 2025. PMID: 40012607 Free PMC article.
-
Macrophage response to fibrin structure mediated by Tgm2-dependent mitochondrial mechanosensing.Bioact Mater. 2025 Apr 22;50:382-395. doi: 10.1016/j.bioactmat.2025.04.022. eCollection 2025 Aug. Bioact Mater. 2025. PMID: 40331213 Free PMC article.
References
-
- Sadtler K, Singh A, Wolf MT, Wang X, Pardoll DM, Elisseeff JH. Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nat Rev Mater. 2016;1(7):1–17.
-
- Chen X, Chen J, Huang N. The structure, formation, and effect of plasma protein layer on the blood contact materials: A review. Biosurf Biotribol. 2022;8(1):1–14.
LinkOut - more resources
Full Text Sources
Research Materials

