Biofabrication methods for reconstructing extracellular matrix mimetics
- PMID: 37719085
- PMCID: PMC10500422
- DOI: 10.1016/j.bioactmat.2023.08.018
Biofabrication methods for reconstructing extracellular matrix mimetics
Abstract
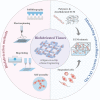
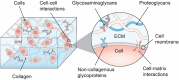
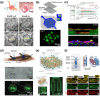
In the human body, almost all cells interact with extracellular matrices (ECMs), which have tissue and organ-specific compositions and architectures. These ECMs not only function as cellular scaffolds, providing structural support, but also play a crucial role in dynamically regulating various cellular functions. This comprehensive review delves into the examination of biofabrication strategies used to develop bioactive materials that accurately mimic one or more biophysical and biochemical properties of ECMs. We discuss the potential integration of these ECM-mimics into a range of physiological and pathological in vitro models, enhancing our understanding of cellular behavior and tissue organization. Lastly, we propose future research directions for ECM-mimics in the context of tissue engineering and organ-on-a-chip applications, offering potential advancements in therapeutic approaches and improved patient outcomes.
Keywords: Biofabrication; Bioprinting; Electrospinning; Extracellular matrix; Organ-on-a-Chip.
© 2023 The Authors.
Conflict of interest statement
Yan Yan Shery Huang is an editorial board member for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Figures





References
-
- Discher D.E., Janmey P., Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. - PubMed
-
- Thery M. Micropatterning as a tool to decipher cell morphogenesis and functions. J. Cell Sci. 2010;123(Pt 24):4201–4213. - PubMed
-
- Lutolf M.P., Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23(1):47–55. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

