Enhanced production of select phytocannabinoids in medical Cannabis cultivars using microbial consortia
- PMID: 37719209
- PMCID: PMC10502174
- DOI: 10.3389/fpls.2023.1219836
Enhanced production of select phytocannabinoids in medical Cannabis cultivars using microbial consortia
Abstract
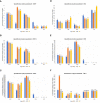
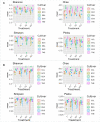
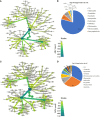
The root microbiome of medical cannabis plants has been largely unexplored due to past legal restrictions in many countries. Microbes that live on and within the tissue of Cannabis sativa L. similar to other plants, provide advantages such as stimulating plant growth, helping it absorb minerals, providing protection against pathogen attacks, and influencing the production of secondary metabolites. To gain insight into the microbial communities of C. sativa cultivars with different tetrahydrocannabinol (THC) and cannabidiol (CBD) profiles, a greenhouse trial was carried out with and without inoculants added to the growth substrate. Illumina MiSeq metabarcoding was used to analyze the root and rhizosphere microbiomes of the five cultivars. Plant biomass production showed higher levels in three of five cultivars inoculated with the arbuscular mycorrhizal fungus Rhizophagus irregularis and microbial suspension. The blossom dry weight of the cultivar THE was greater when inoculated with R. irregularis and microbial suspension than with no inoculation. Increasing plant biomass and blossom dry weight are two important parameters for producing cannabis for medical applications. In mature Cannabis, 12 phytocannabinoid compounds varied among cultivars and were affected by inoculants. Significant differences (p ≤ 0.01) in concentrations of cannabidivarinic acid (CBDVA), cannabidivarin (CBDV), cannabigerol (CBG), cannabidiol (CBD), and cannabigerolic acid (CBGA) were observed in all Cannabis cultivars when amended with F, K1, and K2 inoculants. We found microbes that were shared among cultivars. For example, Terrimicrobium sp., Actinoplanes sp., and Trichoderma reesei were shared by the cultivars ECC-EUS-THE, CCL-ECC, and EUS-THE, respectively. Actinoplanes sp. is a known species that produces phosphatase enzymes, while Trichoderma reesei is a fungal train that produces cellulase and contributes to organic matter mineralization. However, the role of Terrimicrobium sp. as an anaerobic bacterium remains unknown. This study demonstrated that the use of inoculants had an impact on the production of phytocannabinoids in five Cannabis cultivars. These inoculants could have useful applications for optimizing cannabis cultivation practices and increasing the production of phytocannabinoids.
Keywords: Cannabis; arbuscular mycorrhizal fungi; bioinoculant; microbiome; phytocannabinoids; rhizosphere.
Copyright © 2023 Ahmed, Beneš, Hajšlová, Fišarová, Vosátka and Hijri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Anderson M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 26 (1), 32–46.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

