Warmer temperature during asexual reproduction induce methylome, transcriptomic, and lasting phenotypic changes in Fragaria vesca ecotypes
- PMID: 37719273
- PMCID: PMC10500154
- DOI: 10.1093/hr/uhad156
Warmer temperature during asexual reproduction induce methylome, transcriptomic, and lasting phenotypic changes in Fragaria vesca ecotypes
Abstract
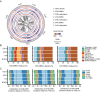
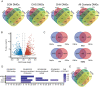
Plants must adapt with increasing speed to global warming to maintain their fitness. One rapid adaptation mechanism is epigenetic memory, which may provide organisms sufficient time to adapt to climate change. We studied how the perennial Fragaria vesca adapted to warmer temperatures (28°C vs. 18°C) over three asexual generations. Differences in flowering time, stolon number, and petiole length were induced by warmer temperature in one or more ecotypes after three asexual generations and persisted in a common garden environment. Induced methylome changes differed between the four ecotypes from Norway, Iceland, Italy, and Spain, but shared methylome responses were also identified. Most differentially methylated regions (DMRs) occurred in the CHG context, and most CHG and CHH DMRs were hypermethylated at the warmer temperature. In eight CHG DMR peaks, a highly similar methylation pattern could be observed between ecotypes. On average, 13% of the differentially methylated genes between ecotypes also showed a temperature-induced change in gene expression. We observed ecotype-specific methylation and expression patterns for genes related to gibberellin metabolism, flowering time, and epigenetic mechanisms. Furthermore, we observed a negative correlation with gene expression when repetitive elements were found near (±2 kb) or inside genes. In conclusion, lasting phenotypic changes indicative of an epigenetic memory were induced by warmer temperature and were accompanied by changes in DNA methylation patterns. Both shared methylation patterns and transcriptome differences between F. vesca accessions were observed, indicating that DNA methylation may be involved in both general and ecotype-specific phenotypic variation.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nanjing Agricultural University.
Conflict of interest statement
None declared.
Figures




Similar articles
-
Major transcriptomic differences are induced by warmer temperature conditions experienced during asexual and sexual reproduction in Fragaria vesca ecotypes.Front Plant Sci. 2023 Jul 14;14:1213311. doi: 10.3389/fpls.2023.1213311. eCollection 2023. Front Plant Sci. 2023. PMID: 37521931 Free PMC article.
-
Methylome, transcriptome, and phenotype changes induced by temperature conditions experienced during sexual reproduction in Fragaria vesca.Physiol Plant. 2023 Jul-Aug;175(4):e13963. doi: 10.1111/ppl.13963. Physiol Plant. 2023. PMID: 37340851
-
Stable Epigenetic Variants Selected from an Induced Hypomethylated Fragaria vesca Population.Front Plant Sci. 2016 Nov 29;7:1768. doi: 10.3389/fpls.2016.01768. eCollection 2016. Front Plant Sci. 2016. PMID: 27965682 Free PMC article.
-
Epigenomics in stress tolerance of plants under the climate change.Mol Biol Rep. 2023 Jul;50(7):6201-6216. doi: 10.1007/s11033-023-08539-6. Epub 2023 Jun 9. Mol Biol Rep. 2023. PMID: 37294468 Review.
-
Environmental Adaptation of Genetically Uniform Organisms with the Help of Epigenetic Mechanisms-An Insightful Perspective on Ecoepigenetics.Epigenomes. 2022 Dec 26;7(1):1. doi: 10.3390/epigenomes7010001. Epigenomes. 2022. PMID: 36648862 Free PMC article. Review.
Cited by
-
The evolutionary consequences of interactions between the epigenome, the genome and the environment.Evol Appl. 2024 Jul 23;17(7):e13730. doi: 10.1111/eva.13730. eCollection 2024 Jul. Evol Appl. 2024. PMID: 39050763 Free PMC article. Review.
-
Global characterization of somatic mutations and DNA methylation changes during vegetative propagation in strawberries.Genome Res. 2024 Oct 29;34(10):1582-1594. doi: 10.1101/gr.279378.124. Genome Res. 2024. PMID: 39406501 Free PMC article.
-
Epigenomic and transcriptomic persistence of heat stress memory in strawberry (Fragaria vesca).BMC Plant Biol. 2024 May 16;24(1):405. doi: 10.1186/s12870-024-05093-6. BMC Plant Biol. 2024. PMID: 38750420 Free PMC article.
References
-
- Basu S. An Insight into the Factors Regulating Flowering in Rice: From Genetics to Epigenetics. In: Roychoudhury A, ed. Rice Research for Quality Improvement: Genomics and Genetic Engineering: Volume 2: Nutrient Biofortification and Herbicide and Biotic Stress Resistance in Rice. Springer: Singapore, 2020,233–47
-
- Forestan C, Farinati S, Zambelli Fet al. . Epigenetic signatures of stress adaptation and flowering regulation in response to extended drought and recovery in Zea mays. Plant Cell Environ. 2020;43:55–75 - PubMed
-
- Agustí M, Mesejo C, Muñoz-Fambuena Net al. . Fruit-dependent epigenetic regulation of flowering in citrus. New Phytol. 2020;225:376–84 - PubMed
-
- Jiang L, Zhang M, Ma K. Whole-genome DNA methylation associated with differentially expressed genes regulated anthocyanin biosynthesis within flower color chimera of ornamental tree Prunus mume. For Trees Livelihoods. 2020;11:90
LinkOut - more resources
Full Text Sources

