Bispecific antibodies in cancer therapy: Target selection and regulatory requirements
- PMID: 37719370
- PMCID: PMC10501874
- DOI: 10.1016/j.apsb.2023.05.023
Bispecific antibodies in cancer therapy: Target selection and regulatory requirements
Abstract
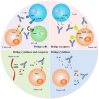
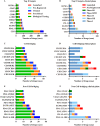
In recent years, the development of bispecific antibodies (bsAbs) has been rapid, with many new structures and target combinations being created. The boom in bsAbs has led to the successive issuance of industry guidance for their development in the US and China. However, there is a high degree of similarity in target selection, which could affect the development of diversity in bsAbs. This review presents a classification of various bsAbs for cancer therapy based on structure and target selection and examines the advantages of bsAbs over monoclonal antibodies (mAbs). Through database research, we have identified the preferences of available bsAbs combinations, suggesting rational target selection options and warning of potential wastage of medical resources. We have also compared the US and Chinese guidelines for bsAbs in order to provide a reference for their development.
Keywords: Bispecific antibody; Cancer immunotherapy; Clinical trials; Mechanism; Oncology; Regulatory guidance; Structure; Target selection.
© 2023 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures




References
-
- Emmons C., Hunsicker L.G. Muromonab-CD3 (orthoclone OKT3): the first monoclonal antibody approved for therapeutic use. Iowa Med. 1987;77:78–82. - PubMed
-
- Torka P., Barth M., Ferdman R., Hernandez-Ilizaliturri F.J. Mechanisms of resistance to monoclonal antibodies (mAbs) in lymphoid malignancies. Curr Hematol Malig Rep. 2019;14:426–438. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

