Bacillus Calmette-Guérin vaccination for protection against recurrent herpes labialis: a nested randomised controlled trial
- PMID: 37719417
- PMCID: PMC10500555
- DOI: 10.1016/j.eclinm.2023.102203
Bacillus Calmette-Guérin vaccination for protection against recurrent herpes labialis: a nested randomised controlled trial
Abstract
Background: Recurrences of herpes simplex virus (HSV) in the orofacial region (herpes labialis or cold sores) impact quality-of-life. We aimed to study whether the bacille Calmette-Guérin (BCG) vaccine can attenuate cold sore recurrences through off-target immunomodulatory effects.
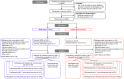
Methods: In this nested randomised controlled trial within the multicentre, phase 3 BRACE trial, 6828 healthcare workers were randomised in 36 sites in Australia, the Netherlands, Spain, the United Kingdom and Brazil, to receive BCG-Denmark or no BCG (1:1 ratio using a web-based procedure) and followed for 12 months with 3-monthly questionnaires. Exclusion criteria included contraindication to BCG vaccine or previous vaccination with BCG within the past year, any other live-attenuated vaccine within the last month, or any COVID-specific vaccine. The intervention group received one intradermal dose of 0.1 mL of BCG-Denmark corresponding to 2-8 x 105 colony forming units of Mycobacterium bovis, Danish strain 1331. The primary outcome was the difference in restricted mean survival time (i.e., time to first cold-sore recurrence), in participants with frequent recurrent herpes labialis (≥4 recurrences/year), analysed by intention-to-treat. Secondary outcomes addressed additional questions, including analyses in other sub-populations. Adverse events were monitored closely during the first 3 months and were reported in all participants who received one dose of study drug according to intervention received. The BRACE trial is registered with ClinicalTrials.gov, NCT04327206.
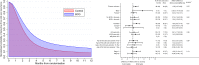
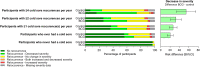
Findings: Between March 30, 2020 and February 18, 2021, 84 individuals with frequent recurrent cold sores were randomly assigned to BCG (n = 38) or control (n = 46). The average time to first cold-sore recurrence was 1.55 months longer in the BCG group (95% CI 0.27-2.82, p = 0.02) than the control group (hazard ratio 0.54, 95% CI 0.32-0.91; intention-to-treat). The beneficial effect of BCG was greater in the as-treated population (difference 1.91 months, 95% CI 0.69-3.12, p = 0.003; hazard ratio 0.45, 95% CI 0.26-0.76). In prespecified subgroup analyses, only sex modified the treatment effect (interaction p = 0.007), with benefit restricted to males. Over 12 months, a greater proportion of participants in the BCG group compared with the control group reported a decrease in duration (61% vs 21%), severity (74% vs 21%), frequency (55% vs 21%), and impact on quality of life (42% vs 15%) of cold sore recurrences. In participants who had ever had a cold sore, there was also a decrease in self-reported burden of recurrences in the BCG group. In participants who had never had a cold sore, there was an increased risk of a first episode in the BCG group (risk difference 1.4%; 95% CI 0.3-2.6%, p = 0.02). There were no safety concerns.
Interpretation: BCG-Denmark vaccination had a beneficial effect on herpes labialis, particularly in males with frequent recurrences, but may increase the risk of a first cold sore.
Funding: Bill & Melinda Gates Foundation, the Minderoo Foundation, Sarah and Lachlan Murdoch, the Royal Children's Hospital Foundation, Health Services Union NSW, the Peter Sowerby Foundation, SA Health, the Insurance Advisernet Foundation, the NAB Foundation, the Calvert-Jones Foundation, the Modara Pines Charitable Foundation, the UHG Foundation Pty Ltd, Epworth Healthcare, and individual donors.
Keywords: Bacille Calmette-Guérin; Cold sore; Herpes labialis; Herpes simplex virus; Heterologous immunity; Mycobacterium bovis; Non-specific effects; Off-target effects; Prevention; Secondary prophylaxis.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
We declare no competing interests.
Figures
References
-
- Whitley R.J., Roizman B. Herpes simplex virus infections. Lancet. 2001;357(9267):1513–1518. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

