Platelet mitochondria, a potent immune mediator in neurological diseases
- PMID: 37719457
- PMCID: PMC10502307
- DOI: 10.3389/fphys.2023.1210509
Platelet mitochondria, a potent immune mediator in neurological diseases
Abstract
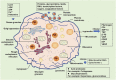
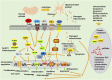
Dysfunction of the immune response is regarded as a prominent feature of neurological diseases, including neurodegenerative diseases, malignant tumors, acute neurotraumatic insult, and cerebral ischemic/hemorrhagic diseases. Platelets play a fundamental role in normal hemostasis and thrombosis. Beyond those normal functions, platelets are hyperactivated and contribute crucially to inflammation and immune responses in the central nervous system (CNS). Mitochondria are pivotal organelles in platelets and are responsible for generating most of the ATP that is used for platelet activation and aggregation (clumping). Notably, platelet mitochondria show marked morphological and functional alterations under heightened inflammatory/oxidative stimulation. Mitochondrial dysfunction not only leads to platelet damage and apoptosis but also further aggravates immune responses. Improving mitochondrial function is hopefully an effective strategy for treating neurological diseases. In this review, the authors discuss the immunomodulatory roles of platelet-derived mitochondria (PLT-mitos) in neurological diseases and summarize the neuroprotective effects of platelet mitochondria transplantation.
Keywords: central nervous system; mitochondria; neuroinflammation; platelet; transplantation.
Copyright © 2023 Ma, Jiang, Yang, Hu, Shen, Shen and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond.Crit Rev Clin Lab Sci. 2016 Dec;53(6):409-30. doi: 10.1080/10408363.2016.1200008. Epub 2016 Jul 22. Crit Rev Clin Lab Sci. 2016. PMID: 27282765 Review.
-
Platelet mitochondrial dysfunction and the correlation with human diseases.Biochem Soc Trans. 2017 Dec 15;45(6):1213-1223. doi: 10.1042/BST20170291. Epub 2017 Oct 20. Biochem Soc Trans. 2017. PMID: 29054925 Review.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Neuro-Immune Hemostasis: Homeostasis and Diseases in the Central Nervous System.Front Cell Neurosci. 2018 Nov 26;12:459. doi: 10.3389/fncel.2018.00459. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30534057 Free PMC article. Review.
-
The molecular basis of platelet biogenesis, activation, aggregation and implications in neurological disorders.Int J Neurosci. 2020 Dec;130(12):1237-1249. doi: 10.1080/00207454.2020.1732372. Epub 2020 Feb 26. Int J Neurosci. 2020. PMID: 32069430 Review.
Cited by
-
Mitochondrial dysfunction is a major cause of thromboinflammation and inflammatory cell death in critical illnesses.Inflamm Res. 2025 Jan 13;74(1):17. doi: 10.1007/s00011-025-01994-w. Inflamm Res. 2025. PMID: 39806233 Review.
-
The Role of Platelet Dysfunctions in the Pathogenesis of the Hemostatic-Coagulant System Imbalances.Int J Mol Sci. 2025 Mar 19;26(6):2756. doi: 10.3390/ijms26062756. Int J Mol Sci. 2025. PMID: 40141398 Free PMC article. Review.
-
Friend or foe: the role of platelets in acute lung injury.Front Immunol. 2025 May 14;16:1556923. doi: 10.3389/fimmu.2025.1556923. eCollection 2025. Front Immunol. 2025. PMID: 40438116 Free PMC article. Review.
-
Mitochondria transfer for myelin repair.J Cereb Blood Flow Metab. 2025 Mar 13:271678X251325805. doi: 10.1177/0271678X251325805. Online ahead of print. J Cereb Blood Flow Metab. 2025. PMID: 40079508 Free PMC article. Review.
-
Platelets and diseases: signal transduction and advances in targeted therapy.Signal Transduct Target Ther. 2025 May 16;10(1):159. doi: 10.1038/s41392-025-02198-8. Signal Transduct Target Ther. 2025. PMID: 40374650 Free PMC article. Review.
References
-
- Abdel-Rahman E. A., Zaky E. A., Aboulsaoud M., Elhossiny R. M., Youssef W. Y., Mahmoud A. M., et al. (2021). Autism spectrum disorder (ASD)-associated mitochondrial deficits are revealed in children's platelets but unimproved by hyperbaric oxygen therapy. Free Radic. Res. 55 (1), 26–40. 10.1080/10715762.2020.1856376 - DOI - PubMed
-
- Acquasaliente L., Pontarollo G., Radu C. M., Peterle D., Artusi I., Pagotto A., et al. (2022). Exogenous human α-Synuclein acts in vitro as a mild platelet antiaggregant inhibiting α-thrombin-induced platelet activation. Sci. Rep. 12 (1), 9880. Published 2022 Jun 14. 10.1038/s41598-022-12886-y - DOI - PMC - PubMed
-
- Amorini A. M., Tuttobene M., Tomasello F. M., Biazzo F., Gullotta S., De Pinto V., et al. (2013). Glucose ameliorates the metabolic profile and mitochondrial function of platelet concentrates during storage in autologous plasma. Blood Transfus. 11 (1), 61–70. 10.2450/2012.0145-11 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

