Mechanical stimuli such as shear stress and piezo1 stimulation generate red blood cell extracellular vesicles
- PMID: 37719461
- PMCID: PMC10502313
- DOI: 10.3389/fphys.2023.1246910
Mechanical stimuli such as shear stress and piezo1 stimulation generate red blood cell extracellular vesicles
Abstract
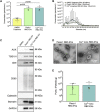
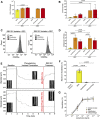
Introduction: Generating physiologically relevant red blood cell extracellular vesicles (RBC-EVs) for mechanistic studies is challenging. Herein, we investigated how to generate and isolate high concentrations of RBC-EVs in vitro via shear stress and mechanosensitive piezo1 ion channel stimulation. Methods: RBC-EVs were generated by applying shear stress or the piezo1-agonist yoda1 to RBCs. We then investigated how piezo1 RBC-EV generation parameters (hematocrit, treatment time, treatment dose), isolation methods (membrane-based affinity, ultrafiltration, ultracentrifugation with and without size exclusion chromatography), and storage conditions impacted RBC-EV yield and purity. Lastly, we used pressure myography to determine how RBC-EVs isolated using different methods affected mouse carotid artery vasodilation. Results: Our results showed that treating RBCs at 6% hematocrit with 10 µM yoda1 for 30 min and isolating RBC-EVs via ultracentrifugation minimized hemolysis, maximized yield and purity, and produced the most consistent RBC-EV preparations. Co-isolated contaminants in impure samples, but not piezo1 RBC-EVs, induced mouse carotid artery vasodilation. Conclusion: This work shows that RBC-EVs can be generated through piezo1 stimulation and may be generated in vivo under physiologic flow conditions. Our studies further emphasize the importance of characterizing EV generation and isolation parameters before using EVs for mechanistic analysis since RBC-EV purity can impact functional outcomes.
Keywords: exosomes; extracellular vesicles; piezo1; red blood cells; shear stress.
Copyright © 2023 Sangha, Weber, Sapp, Setua, Thangaraju, Pettebone, Rogers, Doctor, Buehler and Clyne.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

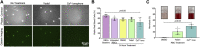


Similar articles
-
Piezo1 regulates shear-dependent nitric oxide production in human erythrocytes.Am J Physiol Heart Circ Physiol. 2022 Jul 1;323(1):H24-H37. doi: 10.1152/ajpheart.00185.2022. Epub 2022 May 13. Am J Physiol Heart Circ Physiol. 2022. PMID: 35559724
-
An Isolation System to Collect High Quality and Purity Extracellular Vesicles from Serum.Int J Nanomedicine. 2021 Sep 29;16:6681-6692. doi: 10.2147/IJN.S328325. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34616151 Free PMC article.
-
Dual action of Dooku1 on PIEZO1 channel in human red blood cells.Front Physiol. 2023 Jul 10;14:1222983. doi: 10.3389/fphys.2023.1222983. eCollection 2023. Front Physiol. 2023. PMID: 37492641 Free PMC article.
-
Extracellular Vesicles in Red Blood Cell Concentrates: An Overview.Transfus Med Rev. 2019 Apr;33(2):125-130. doi: 10.1016/j.tmrv.2019.02.002. Epub 2019 Feb 23. Transfus Med Rev. 2019. PMID: 30910256 Review.
-
Isolation of extracellular vesicles with combined enrichment methods.J Chromatogr B Analyt Technol Biomed Life Sci. 2021 Apr 15;1169:122604. doi: 10.1016/j.jchromb.2021.122604. Epub 2021 Feb 27. J Chromatogr B Analyt Technol Biomed Life Sci. 2021. PMID: 33713953 Review.
Cited by
-
Physicochemical Modulation Strategies for Mass Production of Extracellular Vesicle.Tissue Eng Regen Med. 2025 Jul;22(5):569-591. doi: 10.1007/s13770-025-00726-9. Epub 2025 Jun 5. Tissue Eng Regen Med. 2025. PMID: 40471522 Free PMC article. Review.
-
Mesenchymal stem cell extracellular vesicle vascularization bioactivity and production yield are responsive to cell culture substrate stiffness.Bioeng Transl Med. 2025 Jan 7;10(3):e10743. doi: 10.1002/btm2.10743. eCollection 2025 May. Bioeng Transl Med. 2025. PMID: 40385541 Free PMC article.
-
Extracellular Vesicles as New Players in Drug Delivery: A Focus on Red Blood Cells-Derived EVs.Pharmaceutics. 2023 Jan 21;15(2):365. doi: 10.3390/pharmaceutics15020365. Pharmaceutics. 2023. PMID: 36839687 Free PMC article. Review.
-
Efficient and highly reproducible production of red blood cell-derived extracellular vesicle mimetics for the loading and delivery of RNA molecules.Sci Rep. 2024 Jun 25;14(1):14610. doi: 10.1038/s41598-024-65623-y. Sci Rep. 2024. PMID: 38918594 Free PMC article.
-
Aberrant GPA expression and regulatory function of red blood cells in sickle cell disease.Blood Adv. 2024 Apr 9;8(7):1687-1697. doi: 10.1182/bloodadvances.2023011611. Blood Adv. 2024. PMID: 38231087 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

