Effect of Arsenate and p-Phenylenediamine on the Expression of Aquaporins in Cultured Human Urothelial Cells
- PMID: 37719549
- PMCID: PMC10504450
- DOI: 10.7759/cureus.43606
Effect of Arsenate and p-Phenylenediamine on the Expression of Aquaporins in Cultured Human Urothelial Cells
Abstract
Background: Exposure to arsenic (As) or p‑phenylenediamine (PPD) can lead to dysfunction, or even cancer, in various types of organs, including the urinary bladder, yet the underlying mechanisms remain unclear. Aquaporins (AQPs) are widely expressed small water channel proteins that provide the major route for the transport of water and other small molecules across plasma membranes in diverse cell types. Altered expression of AQPs has been associated with pathologies in all major organs, including the urinary bladder.
Objective: The present in vitro study was performed as a first step towards exploring the possible involvement of AQPs in As- and PPD‑induced bladder diseases.
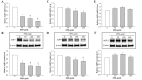
Methods: An immortalized normal human urothelial cell line was employed. Cells were exposed to different concentrations of sodium arsenate (0‑20 μM) or PPD (0‑200 μM) for 48 h. Cell viability was subsequently assessed. The mRNA and protein expression levels of AQPs (specifically, AQP3, 4, 7, 9, and 11) were analyzed using reverse transcription‑quantitative polymerase chain reaction and Western blot analyses, respectively.
Results: The viability of the cells was decreased in a concentration-dependent manner upon exposure to arsenate. The mRNA and protein expression levels of AQP3, 4, 7, and 9 were substantially reduced, whereas the expression of AQP11 was largely unchanged. As for the experiments with PPD, treatment with increasing concentrations of PPD induced a gradual decrease in cell viability. The mRNA and protein expression levels of AQP3, 4, and 11 were generally unaltered; however, a marked reduction in the expression levels of AQP7 was observed, contrasting with a gradual concentration-dependent decrease in the expression of AQP9.
Conclusion: The importance of the differential expression profiles of the AQPs induced by arsenate and PPD requires further investigation; nevertheless, the findings of the present study suggest that AQPs have a role in As‑ and PPD‑induced bladder diseases.
Keywords: aquaporin; arsenate; p-phenylenediamine; urinary bladder; urothelial cell.
Copyright © 2023, Wu et al.
Conflict of interest statement
The authors have declared financial relationships, which are detailed in the next section.
Figures



References
-
- Arsenic methylation, urinary arsenic metabolites and human diseases: current perspective. Tseng CH. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25:1–22. - PubMed
-
- Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Rahaman MS, Rahman MM, Mise N, et al. Environ Pollut. 2021;289:117940. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
