Effect of Platelet Parameters on Linezolid-Related Thrombocytopenia in Hospitalized Patients
- PMID: 37719650
- PMCID: PMC10505032
- DOI: 10.2147/IDR.S408102
Effect of Platelet Parameters on Linezolid-Related Thrombocytopenia in Hospitalized Patients
Abstract
Background: Linezolid-induced thrombocytopenia incidence varies considerably. Linezolid-related thrombocytopenia in patients has received few studies which have investigated risk factors including platelet parameters except for platelet counts. The study aims to analyze the effect of platelet parameters, including mean platelet volume and platelet large cell ratio, on linezolid-related thrombocytopenia in patients.
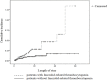
Methods: The effect of platelet parameters on linezolid-related thrombocytopenia was identified by univariate and multivariate logistic regressions. A Kaplan-Meier survival analysis was carried out to compare the survival of patients who developed linezolid-related thrombocytopenia with patients who did not.
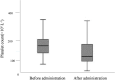
Results: Thrombocytopenia occurred at a rate of 41.5% (66/159) after linezolid therapy in hospitalized patients. Platelet parameters, including the difference in mean platelet volume (MPV/fL=0.08 (-1.2-0.9)vs-0.5 (-1.5-0.3), (OR, 0.459; P = 0.001), the difference in platelet large cell ratio (PLCR/fL=0.9 (-5.1-6.2)vs-3.8 (-8.6-2.4), (OR, 1.156; P = 0.001), baseline platelet counts (OR, 0.995; P = 0.006) and duration of linezolid therapy≥10d (OR, 1.346; P = 0.007), were significantly associated with linezolid-related thrombocytopenia in hospitalized patients. In addition, other risk factors which also are associated with linezolid-related thrombocytopenia include baseline red blood cells, co-medication with parecoxib and co-medication with caspofungin. Accumulated in-hospital mortality of patients with thrombocytopenia was significantly higher than that of patients without thrombocytopenia during linezolid treatment (19.7% vs 8.6%, P = 0.003).
Conclusion: The difference in mean platelet volume, the difference in large platelet ratio, baseline platelet counts and duration of linezolid therapy≥10d significantly affected the development of linezolid-related thrombocytopenia in hospitalized patients.
Keywords: linezolid; platelet parameters; risk factors; thrombocytopenia.
© 2023 Zhang et al.
Conflict of interest statement
The authors declare that there is no conflict of interest in this work.
Figures
Similar articles
-
Retrospective cohort chart review study of factors associated with the development of thrombocytopenia in adult Japanese patients who received intravenous linezolid therapy.Clin Ther. 2009 Oct;31(10):2126-33. doi: 10.1016/j.clinthera.2009.10.017. Clin Ther. 2009. PMID: 19922883
-
Retrospective analysis of the risk factors for linezolid-induced thrombocytopenia in adult Japanese patients.Int J Clin Pharm. 2014 Aug;36(4):795-9. doi: 10.1007/s11096-014-9961-6. Epub 2014 Jun 10. Int J Clin Pharm. 2014. PMID: 24913359
-
Linezolid-induced thrombocytopenia increases mortality risk in intensive care unit patients, a 10 year retrospective study.J Clin Pharm Ther. 2019 Feb;44(1):84-90. doi: 10.1111/jcpt.12762. Epub 2018 Sep 22. J Clin Pharm Ther. 2019. PMID: 30243033
-
External Evaluation of Population Pharmacokinetics and Pharmacodynamics in Linezolid-Induced Thrombocytopenia: The Transferability of Published Models to Different Hospitalized Patients.Ther Drug Monit. 2021 Apr 1;43(2):271-278. doi: 10.1097/FTD.0000000000000816. Ther Drug Monit. 2021. PMID: 33009290
-
Hematological toxicities associated with linezolid therapy in adults: key findings and clinical considerations.Expert Rev Clin Pharmacol. 2023 Mar;16(3):219-230. doi: 10.1080/17512433.2023.2181160. Epub 2023 Feb 21. Expert Rev Clin Pharmacol. 2023. PMID: 36787631 Review.
Cited by
-
Coagulation dysfunction events associated with echinocandins: a real-world study from FDA adverse event reporting system (FAERS) database.Thromb J. 2024 Aug 23;22(1):78. doi: 10.1186/s12959-024-00641-4. Thromb J. 2024. PMID: 39180077 Free PMC article.
-
Using Machine Learning to Predict Linezolid-Associated Thrombocytopenia.Infect Drug Resist. 2025 May 23;18:2653-2661. doi: 10.2147/IDR.S479658. eCollection 2025. Infect Drug Resist. 2025. PMID: 40438533 Free PMC article.
-
Retrospective Analysis of Risk Factors for Cefoperazone/Sulbactam-Induced Thrombocytopenia in Adult Chinese Patients: A Six-Year Real-World Study.Infect Drug Resist. 2024 Sep 5;17:3901-3911. doi: 10.2147/IDR.S475590. eCollection 2024. Infect Drug Resist. 2024. PMID: 39253607 Free PMC article.
References
-
- Lazaris A, Coleman DC, Kearns AM, et al. Novel multiresistance cfr plasmids in linezolid-resistant methicillin-resistant Staphylococcus epidermidis and vancomycin-resistant Enterococcus faecium (VRE) from a hospital outbreak: co-location of cfr and optrA in VRE. J Antimicrob Chemother. 2017;72(12):3252–3257. doi:10.1093/jac/dkx292 - DOI - PubMed
-
- Dong HY, Xie J, Chen LH, et al. Therapeutic drug monitoring and receiver operating characteristic curve prediction may reduce the development of linezolid-associated thrombocytopenia in critically ill patients. Eur J Clin Microbiol Infect Dis. 2014;33(6):1029–1035. doi:10.1007/s10096-013-2041-3 - DOI - PubMed
LinkOut - more resources
Full Text Sources