Aggregatibacter actinomycetemcomitans cytolethal distending toxin modulates host phagocytic function
- PMID: 37719670
- PMCID: PMC10500838
- DOI: 10.3389/fcimb.2023.1220089
Aggregatibacter actinomycetemcomitans cytolethal distending toxin modulates host phagocytic function
Erratum in
-
Corrigendum: Aggregatibacter actinomycetemcomitans cytolethal distending toxin modulates host phagocytic function.Front Cell Infect Microbiol. 2023 Oct 25;13:1321218. doi: 10.3389/fcimb.2023.1321218. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37965259 Free PMC article.
Abstract
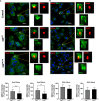
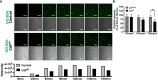
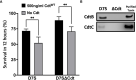
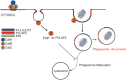
Cytolethal distending toxins (Cdt) are a family of toxins produced by several human pathogens which infect mucocutaneous tissue and induce inflammatory disease. Human macrophages exposed to Aggregatibacter actinomycetemcomitans (Aa) Cdt respond through canonical and non-canonical inflammasome activation to stimulate cytokine release. The inflammatory response is dependent on PI3K signaling blockade via the toxin's phosphatidylinositol-3,4,5-triphosphate (PIP3) phosphatase activity; converting PIP3 to phosphatidylinsoitol-3,4-diphosphate (PI3,4P2) thereby depleting PIP3 pools. Phosphoinositides, also play a critical role in phagosome trafficking, serving as binding domains for effector proteins during phagosome maturation and subsequent fusion with lysosomes. We now demonstrate that AaCdt manipulates the phosphoinositide (PI) pools of phagosome membranes and alters Rab5 association. Exposure of macrophages to AaCdt slowed phagosome maturation and decreased phago-lysosome formation, thereby compromising macrophage phagocytic function. Moreover, macrophages exposed to Cdt showed decreased bactericidal capacity leading to increase in Aggregatibacter actinomycetemcomitans survival. Thus, Cdt may contribute to increased susceptibility to bacterial infection. These studies uncover an underexplored aspect of Cdt function and provide new insight into the virulence potential of Cdt in mediating the pathogenesis of disease caused by Cdt-producing organisms such as Aa.
Keywords: Aggregatibacter actinomycetemcomitans; Cytolethal distending toxin; localized aggressive periodontitis; phagocytosis; phagosome maturation; phosphoinositide.
Copyright © 2023 Kim, Shenker, MacElory, Spradlin, Walker and Boesze-Battaglia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Comment in
-
Editorial: Innate immune evasion strategies during microbial infection.Front Cell Infect Microbiol. 2023 Nov 10;13:1332253. doi: 10.3389/fcimb.2023.1332253. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38029251 Free PMC article. No abstract available.
Similar articles
-
The cytolethal distending toxin of Aggregatibacter actinomycetemcomitans inhibits macrophage phagocytosis and subverts cytokine production.Cytokine. 2014 Mar;66(1):46-53. doi: 10.1016/j.cyto.2013.12.014. Epub 2014 Jan 14. Cytokine. 2014. PMID: 24548424
-
Blockade of the PI-3K signalling pathway by the Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces macrophages to synthesize and secrete pro-inflammatory cytokines.Cell Microbiol. 2014 Sep;16(9):1391-404. doi: 10.1111/cmi.12299. Epub 2014 May 1. Cell Microbiol. 2014. PMID: 24697951 Free PMC article.
-
Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin-Induces Cell Cycle Arrest in a Glycogen Synthase Kinase (GSK)-3-Dependent Manner in Oral Keratinocytes.Int J Mol Sci. 2022 Oct 5;23(19):11831. doi: 10.3390/ijms231911831. Int J Mol Sci. 2022. PMID: 36233133 Free PMC article.
-
A Journey of Cytolethal Distending Toxins through Cell Membranes.Front Cell Infect Microbiol. 2016 Aug 10;6:81. doi: 10.3389/fcimb.2016.00081. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27559534 Free PMC article. Review.
-
Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis.Mol Oral Microbiol. 2016 Jun;31(3):207-27. doi: 10.1111/omi.12119. Epub 2015 Sep 22. Mol Oral Microbiol. 2016. PMID: 26197893 Free PMC article. Review.
Cited by
-
Innovative strategies targeting oral microbial dysbiosis: unraveling mechanisms and advancing therapies for periodontitis.Front Cell Infect Microbiol. 2025 Apr 30;15:1556688. doi: 10.3389/fcimb.2025.1556688. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40370404 Free PMC article. Review.
-
Helicobacter mastomyrinus infection induces autoimmune hepatitis in mice.J Transl Autoimmun. 2025 Jan 24;10:100275. doi: 10.1016/j.jtauto.2025.100275. eCollection 2025 Jun. J Transl Autoimmun. 2025. PMID: 39981114 Free PMC article.
-
Advances in the study of the relationship between Porphyromonas gingivalis and various diseases.Front Cell Dev Biol. 2025 Aug 6;13:1480233. doi: 10.3389/fcell.2025.1480233. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40843171 Free PMC article. Review.
-
Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin Induces Cellugyrin-(Synaptogyrin 2) Dependent Cellular Senescence in Oral Keratinocytes.Pathogens. 2024 Feb 8;13(2):155. doi: 10.3390/pathogens13020155. Pathogens. 2024. PMID: 38392893 Free PMC article.
-
A Synthetic Small Molecule, LGM2605: A Promising Modulator of Increased Pro-Inflammatory Cytokine and Osteoclast Differentiation by Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin.Dent J (Basel). 2024 Jun 26;12(7):195. doi: 10.3390/dj12070195. Dent J (Basel). 2024. PMID: 39056982 Free PMC article.
References
-
- Ando-Suguimoto E. S., da Silva M. P., Kawamoto D., Chen C., DiRienzo J. M., Mayer M. P.. (2014). The cytolethal distending toxin of Aggregatibacter actinomycetemcomitans inhibits macrophage phagocytosis and subverts cytokine production. Cytokine 66 (1), 46–53. doi: 10.1016/j.cyto.2013.12.014 - DOI - PubMed
-
- Boesze-Battaglia K., Walker L. P., Dhingra A., Kandror K., Tang H. Y., Shenker B. J. (2017). Internalization of the Active Subunit of the Aggregatibacter actinomycetemcomitans Cytolethal Distending Toxin Is Dependent upon Cellugyrin (Synaptogyrin 2), a Host Cell Non-Neuronal Paralog of the Synaptic Vesicle Protein, Synaptogyrin 1. Front. Cell Infect. Microbiol. 7, 469. doi: 10.3389/fcimb.2017.00469 - DOI - PMC - PubMed
-
- Boesze-Battaglia K., Dhingra A., Walker L. M., Zekavat A., Shenker B. J. (2020). Internalization and intoxication of human macrophages by the active subunit of the aggregatibacter actinomycetemcomitans cytolethal distending toxin is dependent upon cellugyrin (Synaptogyrin-2). Front. Immunol. 11, 1262. doi: 10.3389/fimmu.2020.01262 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

