Hybrid microneedle arrays for antibiotic and near-IR photothermal synergistic antimicrobial effect against Methicillin-Resistant Staphylococcus aureus
- PMID: 37719675
- PMCID: PMC7615096
- DOI: 10.1016/j.cej.2023.142127
Hybrid microneedle arrays for antibiotic and near-IR photothermal synergistic antimicrobial effect against Methicillin-Resistant Staphylococcus aureus
Abstract
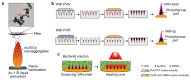
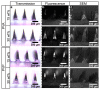
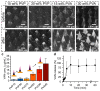
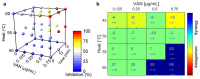
The rise of antibiotic-resistant skin and soft tissue infections (SSTIs) necessitates the development of novel treatments to improve the efficiency and delivery of antibiotics. The incorporation of photothermal agents such as plasmonic nanoparticles (NPs) improves the antibacterial efficiency of antibiotics through synergism with elevated temperatures. Hybrid microneedle (MN) arrays are promising local delivery platforms that enable co-therapy with therapeutic and photothermal agents. However, to-date, the majority of hybrid MNs have focused on the potential treatment of skin cancers, while suffering from the shortcoming of the intradermal release of photothermal agents. Here, we developed hybrid, two-layered MN arrays consisting of an outer water-soluble layer loaded with vancomycin (VAN) and an inner water-insoluble near-IR photothermal core. The photothermal core consists of flame-made plasmonic Au/SiO2 nanoaggregates and polymethylmethacrylate (PMMA). We analyzed the effect of the outer layer polymer, polyvinyl alcohol (PVA) and polyvinylpyrrolidone (PVP), on MN morphology and performance. Hybrid MNs produced with 30 wt% PVA contain a highly drug-loaded outer shell allowing for the incorporation of VAN concentrations up to 100 mg g-1 and temperature increases up to 60 °C under near-IR irradiation while showing sufficient mechanical strength for skin insertion. Furthermore, we studied the combinatorial effect of VAN and heat on the growth inhibition of methicillin-resistant Staphylococcus aureus (MRSA) showing synergistic inhibition between VAN and heat above 55 °C for 10 min. Finally, we show that treatment with hybrid MN arrays can inhibit the growth of MRSA due to the synergistic interaction of heat with VAN reducing the bacterial survival by up to 80%. This proof-of-concept study demonstrates the potential of hybrid, two-layered MN arrays as a novel treatment option for MRSA-associated skin infections.
Keywords: Combination treatment; Gold nanoparticles; Local delivery; MRSA; Skin patch; Thermotherapy.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Vancomycin-Loaded Microneedle Arrays against Methicillin-Resistant Staphylococcus Aureus Skin Infections.Adv Mater Technol. 2021 Jul;6(7):2001307. doi: 10.1002/admt.202001307. Epub 2021 May 5. Adv Mater Technol. 2021. PMID: 34307835 Free PMC article.
-
Customizable Fabrication of Photothermal Microneedles with Plasmonic Nanoparticles Using Low-Cost Stereolithography Three-Dimensional Printing.ACS Appl Bio Mater. 2024 Jul 15;7(7):4533-4541. doi: 10.1021/acsabm.4c00411. Epub 2024 Jun 15. ACS Appl Bio Mater. 2024. PMID: 38877987 Free PMC article.
-
Highly Efficient Near-IR Photothermal Microneedles with Flame-Made Plasmonic Nanoaggregates for Reduced Intradermal Nanoparticle Deposition.Adv Mater Interfaces. 2022 Oct 10;9(34):admi.202201540. doi: 10.1002/admi.202201540. eCollection 2022 Dec. Adv Mater Interfaces. 2022. PMID: 37720386 Free PMC article.
-
Topical Nanotherapeutics for Treating MRSA-Associated Skin and Soft Tissue Infection (SSTIs).AAPS PharmSciTech. 2023 Apr 26;24(5):108. doi: 10.1208/s12249-023-02563-2. AAPS PharmSciTech. 2023. PMID: 37100956 Review.
-
Treatment options for uncomplicated community-acquired skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus: oral antimicrobial agents.Surg Infect (Larchmt). 2008;9 Suppl 1:s29-34. doi: 10.1089/sur.2008.065.supp. Surg Infect (Larchmt). 2008. PMID: 18844472 Review.
Cited by
-
Trends in Photothermal Nanostructures for Antimicrobial Applications.Int J Mol Sci. 2023 May 27;24(11):9375. doi: 10.3390/ijms24119375. Int J Mol Sci. 2023. PMID: 37298326 Free PMC article. Review.
-
Magnetic microfiber hyperthermia for synergistic antimicrobial activity against methicillin-resistant Staphylococcus aureus.Mater Today Bio. 2025 May 12;32:101862. doi: 10.1016/j.mtbio.2025.101862. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40487155 Free PMC article.
-
Emerging Trends in Dissolving-Microneedle Technology for Antimicrobial Skin-Infection Therapies.Pharmaceutics. 2024 Sep 8;16(9):1188. doi: 10.3390/pharmaceutics16091188. Pharmaceutics. 2024. PMID: 39339224 Free PMC article. Review.
-
Preparation and Functionalization of Polymers with Antibacterial Properties-Review of the Recent Developments.Materials (Basel). 2023 Jun 15;16(12):4411. doi: 10.3390/ma16124411. Materials (Basel). 2023. PMID: 37374596 Free PMC article. Review.
-
A Self-Adhesive Ginsenoside Rk3/Metformin-Loaded Hydrogel Microneedle for Management of Systemic Sclerosis.Gels. 2025 May 23;11(6):384. doi: 10.3390/gels11060384. Gels. 2025. PMID: 40558683 Free PMC article.
References
-
- Zeeuwen PLJM, Boekhorst J, van den Bogaard EH, de Koning HD, van de Kerkhof PMC, Saulnier DM, van Swam II, van Hijum SAFT, Kleerebezem M, Schalkwijk J, Timmerman HM. Microbiome dynamics of human epidermis following skin barrier disruption. Genome Biol. 2012;13:R101. doi: 10.1186/gb-2012-13-11-r101. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
