Impact of the progressive uptake of pneumococcal conjugate vaccines on the epidemiology and antimicrobial resistance of invasive pneumococcal disease in Gipuzkoa, northern Spain, 1998-2022
- PMID: 37719737
- PMCID: PMC10501722
- DOI: 10.3389/fpubh.2023.1238502
Impact of the progressive uptake of pneumococcal conjugate vaccines on the epidemiology and antimicrobial resistance of invasive pneumococcal disease in Gipuzkoa, northern Spain, 1998-2022
Abstract
Objectives: To analyze the impact of pneumococcal conjugate vaccines (PCVs) on the incidence of invasive pneumococcal diseases (IPDs) and pneumococcal antibiotic resistance in Gipuzkoa, northern Spain for a 25 years period.
Methods: All cases of IPD confirmed by culture between 1998 and 2022 in a population of around 427,416 people were included. Pneumococci were serotyped and antimicrobial susceptibility was assessed by the EUCAST guidelines.
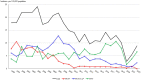
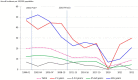
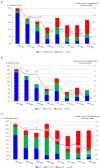
Results: Overall, 1,516 S. pneumoniae isolates were collected. Annual IPD incidence rates (per 100,000 people) declined from 19.9 in 1998-2001 to 11.5 in 2017-19 (42.2% reduction), especially in vaccinated children (from 46.7 to 24.9) and non-vaccinated older adult individuals (from 48.0 to 23.6). After PCV13 introduction, the decrease in the incidence of infections caused by PCV13 serotypes was balanced by the increase in the incidence of non-PCV13 serotypes. In the pandemic year of 2020, IPD incidence was the lowest: 2.81. The annual incidence rates of penicillin-resistant isolates also decreased, from 4.91 in 1998-2001 to 1.49 in 2017-19 and 0.70 in 2020. Since 2017, serotypes 14, 19A, and 11A have been the most common penicillin-resistant types. The incidence of erythromycin-resistant strains declined, from 3.65 to 1.73 and 0.70 in the same years.
Conclusion: PCV use was associated with declines in the incidence of IPD and the spread of non-vaccine serotypes, that balanced the beneficial effect off PCV13, some of them showing high rates of antibiotic resistance.
Keywords: SARS-CoV-2 preventive measures; invasive pneumococcal disease; non-vaccine serotypes; pneumococcal antibiotic resistance; pneumococcal conjugate vaccines.
Copyright © 2023 Manzanal, Vicente, Alonso, Azkue, Ercibengoa and Marimón.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al. . Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente vaccine study center group. Pediatr Infect Dis J. (2000) 19:187–95. doi: 10.1097/00006454-200003000-00003, PMID: - DOI - PubMed
-
- Palmu AA, Jokinen J, Borys D, Nieminen H, Ruokokoski E, Siira L, et al. . Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet. (2013) 381:214–22. doi: 10.1016/S0140-6736(12)61854-6, PMID: - DOI - PubMed
-
- Lo SW, Gladstone RA, van Tonder AJ, Lees JA, du Plessis M, Benisty R, et al. . Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: an international whole-genome sequencing study. Lancet Infect Dis. (2019) 19:759–69. doi: 10.1016/S1473-3099(19)30297-X, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

