Role of estrogen in the regulation of central and peripheral energy homeostasis: from a menopausal perspective
- PMID: 37719789
- PMCID: PMC10504839
- DOI: 10.1177/20420188231199359
Role of estrogen in the regulation of central and peripheral energy homeostasis: from a menopausal perspective
Abstract
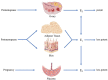
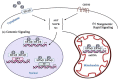
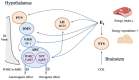
Estrogen plays a prominent role in regulating and coordinating energy homeostasis throughout the growth, development, reproduction, and aging of women. Estrogen receptors (ERs) are widely expressed in the brain and nearly all tissues of the body. Within the brain, central estrogen via ER regulates appetite and energy expenditure and maintains cell glucose metabolism, including glucose transport, aerobic glycolysis, and mitochondrial function. In the whole body, estrogen has shown beneficial effects on weight control, fat distribution, glucose and insulin resistance, and adipokine secretion. As demonstrated by multiple in vitro and in vivo studies, menopause-related decline of circulating estrogen may induce the disturbance of metabolic signals and a significant decrease in bioenergetics, which could trigger an increased incidence of late-onset Alzheimer's disease, type 2 diabetes mellitus, hypertension, and cardiovascular diseases in postmenopausal women. In this article, we have systematically reviewed the role of estrogen and ERs in body composition and lipid/glucose profile variation occurring with menopause, which may provide a better insight into the efficacy of hormone therapy in maintaining energy metabolic homeostasis and hold a clue for development of novel therapeutic approaches for target tissue diseases.
Keywords: energy homeostasis; estrogen; glucose; lipid; menopause; obesity.
© The Author(s), 2023.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
Estrogen: a master regulator of bioenergetic systems in the brain and body.Front Neuroendocrinol. 2014 Jan;35(1):8-30. doi: 10.1016/j.yfrne.2013.08.001. Epub 2013 Aug 29. Front Neuroendocrinol. 2014. PMID: 23994581 Free PMC article. Review.
-
Tetragonia tetragonioides (Pall.) Kuntze protects estrogen-deficient rats against disturbances of energy and glucose metabolism and decreases proinflammatory cytokines.Exp Biol Med (Maywood). 2017 Mar;242(6):593-605. doi: 10.1177/1535370216683835. Epub 2016 Dec 13. Exp Biol Med (Maywood). 2017. PMID: 28241734 Free PMC article.
-
Metabolism Regulation by Estrogens and Their Receptors in the Central Nervous System Before and After Menopause.Horm Metab Res. 2016 Aug;48(8):489-96. doi: 10.1055/s-0042-110320. Epub 2016 Jul 8. Horm Metab Res. 2016. PMID: 27392117 Review.
-
Menopause, central body fatness, and insulin resistance: effects of hormone-replacement therapy.Coron Artery Dis. 1998;9(8):503-11. doi: 10.1097/00019501-199809080-00006. Coron Artery Dis. 1998. PMID: 9847982 Review.
-
Crosstalk between the muscular estrogen receptor α and BDNF/TrkB signaling alleviates metabolic syndrome via 7,8-dihydroxyflavone in female mice.Mol Metab. 2021 Mar;45:101149. doi: 10.1016/j.molmet.2020.101149. Epub 2020 Dec 19. Mol Metab. 2021. PMID: 33352311 Free PMC article.
Cited by
-
Estrogen deficiency in the menopause and the role of hormone therapy: integrating the findings of basic science research with clinical trials.Menopause. 2024 Oct 1;31(10):926-939. doi: 10.1097/GME.0000000000002407. Epub 2024 Jul 30. Menopause. 2024. PMID: 39081162 Free PMC article. Review.
-
Menopause Hot Flashes and Molecular Mechanisms Modulated by Food-Derived Nutrients.Nutrients. 2024 Feb 26;16(5):655. doi: 10.3390/nu16050655. Nutrients. 2024. PMID: 38474783 Free PMC article. Review.
-
Clinical Features and Analysis in Pituitary Stalk Interruption Syndrome.Int J Endocrinol. 2024 May 24;2024:2493083. doi: 10.1155/2024/2493083. eCollection 2024. Int J Endocrinol. 2024. PMID: 38828392 Free PMC article.
-
Deciphering the role of classical oestrogen receptor in insulin resistance and type 2 diabetes mellitus: From molecular mechanism to clinical evidence.Bioimpacts. 2024 Aug 4;15:30378. doi: 10.34172/bi.30378. eCollection 2025. Bioimpacts. 2024. PMID: 40256228 Free PMC article. Review.
-
Consumption of soya isoflavones improved polycystic ovary syndrome-associated metabolic disorders in a rat model.Br J Nutr. 2024 Jun 3;132(4):1-9. doi: 10.1017/S0007114524001296. Online ahead of print. Br J Nutr. 2024. PMID: 38826091 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

