High Positivity Rate of Urinary Mycobacterium tuberculosis Antigens Cocktail (ESAT-6, CFP-10, and MPT-64) in Active Tuberculosis Patients With and Without Human Immunodeficiency Virus Infection: A Cross-Sectional Study
- PMID: 37719805
- PMCID: PMC10501057
- DOI: 10.1177/2632010X231198831
High Positivity Rate of Urinary Mycobacterium tuberculosis Antigens Cocktail (ESAT-6, CFP-10, and MPT-64) in Active Tuberculosis Patients With and Without Human Immunodeficiency Virus Infection: A Cross-Sectional Study
Abstract
Introduction: Human immunodeficiency virus (HIV) infection is a risk factor for the occurrence of a large of Mycobacterium tuberculosis (Mtb) antigen load in the body. The antigens cocktail namely early secretory antigenic target protein 6-kDa (ESAT-6), Culture filtrate protein 10 kDa (CFP-10), and Mycobacterium tuberculosis protein 64 (MPT-64) are secreted by Mtb during replication, hence, their concentration increase in patients with active Tuberculosis (TB). This increased levels facilitates their entry into the systemic circulation, followed by secretion by the glomerulus into the urine. The aim of this study was to determine the positivity rate of the urinary Mtb antigens cocktail between TB patients with and without HIV infection.
Methods: This is an observational descriptive comparative study conducted with a cross-sectional design. Random urine samples were collected from patients diagnosed with active TB in Dr. Hasan Sadikin Bandung Hospital in 2021. The subjects were divided into 2 groups, TB-HIV group and TB without HIV group. The samples were tested using the quantitative immunochromatography method.
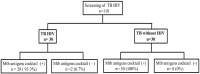
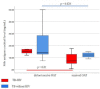
Result: Sixty active TB patients consisting of TB patients with HIV infection (n = 30) and TB patients without HIV infection (n = 30). The positivity in the urinary Mtb antigens cocktail was 93.3% for TB-HIV group and 100% for TB without HIV group (P = .492). The median concentration of urinary Mtb antigens cocktail in TB patients without HIV infection was higher than that of TB patients with HIV infection (137.73 ng/mL vs 96.69 ng/mL, respectively; P = .001).
Conclusion: There was no significant difference in the positivity rate, meanwhile, there was a significant difference in concentration of the urinary Mtb antigens cocktail between active TB patients with and without HIV infection. Interestingly, this urinary Mtb antigens cocktail can be found in both groups without being affected by the patient's immune condition, thus becoming a test to assist diagnose active TB.
Keywords: CFP-10; ESAT-6; HIV; MPT-64; TB; urinary Mtb antigens cocktail.
© The Author(s) 2023.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dewi Kartika Turbawaty is an academic author, a clinical pathologist, and the Head of Clinical Pathology, staff of Microbiology and Biomolecular Department, Faculty of Medicine Padjadjaran University, Dr. Hasan Sadikin General Hospital. Novie Rahmawati Surdjaja is a clinical pathologist. Agnes Rengga Indrati is an academic author, a clinical pathologist, and staff of the Immunoserology Department, Faculty of Medicine Padjadjaran University, Dr. Hasan Sadikin General Hospital. Leni Lismayanti is an academic author, a clinical pathologist, and staff of the Hematology Department, Faculty of Medicine Padjadjaran University, Dr. Hasan Sadikin General Hospital. Verina Logito is an academic author, a clinical pathologist, staff of Immunoserology Department, Faculty of Medicine Padjadjaran University, Dr. Hasan Sadikin General Hospital. The remaining authors declare no relevant financial disclosures or conflicts of interest.
Figures

Similar articles
-
Comparison of the Performance of Urinary Mycobacterium tuberculosis Antigens Cocktail (ESAT6, CFP10, and MPT64) with Culture and Microscopy in Pulmonary Tuberculosis Patients.Int J Microbiol. 2017;2017:3259329. doi: 10.1155/2017/3259329. Epub 2017 Oct 18. Int J Microbiol. 2017. PMID: 29181028 Free PMC article.
-
Generation of Mycobacterium tuberculosis-specific recombinant antigens and evaluation of the clinical value of antibody detection for serological diagnosis of pulmonary tuberculosis.Int J Mol Med. 2013 Mar;31(3):751-7. doi: 10.3892/ijmm.2013.1254. Epub 2013 Jan 22. Int J Mol Med. 2013. PMID: 23338746
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
Increased IgG1, IFN-gamma, TNF-alpha and IL-6 responses to Mycobacterium tuberculosis antigens in patients with tuberculosis are lower after chemotherapy.Int Immunol. 2010 Sep;22(9):775-82. doi: 10.1093/intimm/dxq429. Epub 2010 Jul 11. Int Immunol. 2010. PMID: 20624776
-
[Characteristics of a diagnostic method for tuberculosis infection based on whole blood interferon-gamma assay].Kekkaku. 2006 Nov;81(11):681-6. Kekkaku. 2006. PMID: 17154047 Review. Japanese.
References
-
- Shankar EM, Vignesh R, Ellegård R, et al.. HIV-Mycobacterium tuberculosis co-infection: a ‘danger-couple model’ of disease pathogenesis. Pathog Dis. 2014;70:110-118. - PubMed
-
- World Health Organization. Global Tuberculosis Report 2019. WHO; 2019.
-
- World Health Organization. Guidelines Approved by the Guidelines Review Committee. WHO Consolidated Guidelines on Tuberculosis. Module 3: The Diagnosis – Rapid Diagnostics for Tuberculosis Detection. WHO; 2020.
-
- Turbawaty DK, Subroto H, Verdiansah Soeroto AY, Setiabudiawan B, Parwati I. Validity of Mycobacterium tuberculosis antigens cocktail (ESAT-6, CFP-10, MPT 64) serum test in diagnosing adult and pediatric tuberculosis. Int J Sci Res. 2017:362-366.
LinkOut - more resources
Full Text Sources
Miscellaneous

