Development and Validation of a Clinical Risk Score to Predict Immune-mediated Liver Injury Caused by Sintilimab: Assessed for Causality Using Updated RUCAM
- PMID: 37719962
- PMCID: PMC10500293
- DOI: 10.14218/JCTH.2023.00124
Development and Validation of a Clinical Risk Score to Predict Immune-mediated Liver Injury Caused by Sintilimab: Assessed for Causality Using Updated RUCAM
Abstract
Background and aims: Immune-mediated liver injury is a fatal side effect of sintilimab. This study aimed to shed light on the associated risk factors and characteristics of this adverse event.
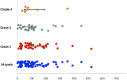
Methods: The clinical records of 772 patients treated with sintilimab were retrospectively reviewed to investigate risk factors associated with sintilimab immune-related hepatotoxicity, as well as its incidence and outcome. The Roussel Uclaf Causality Assessment Method was used to identify cases of sintilimab-induced hepatotoxicity. Furthermore, logistic regressions were performed to compare the clinical and bloodwork characteristics of patients with and without immune-mediated liver injury caused by checkpoint inhibitors.
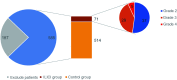
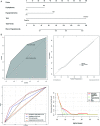
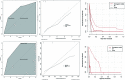
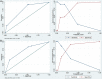
Results: Of the 585 patients included in the study, 71 (12.1%) developed liver injury during sintilimab use. The median RUCAM score with interquartile range was 7 (6, 8). Hypoproteinemia, dyslipidemia, and the presence of thyroid peroxidase antibodies were risk factors for sintilimab-related hepatotoxicity. A nomogram model was constructed for sintilimab-induced immune-mediated liver injury based on these risk factors, which had a C-index value of 0.713 and a good calibration curve. When applied to patients with grade ≥3 and ≥4 sintilimab-induced immune-mediated liver injury, it achieved C-index values of 0.752 and 0.811, respectively. The nomogram model also showed a good prediction potential in patients ≥65 years and males. Six of the patients with sintilimab-related hepatotoxicity showed improved liver function upon treatment with steroids.
Conclusions: This study demonstrated that hypoproteinemia, dyslipidemia, and the presence of thyroid peroxidase antibodies were clinically feasible prognostic biomarkers to predict liver injury in patients treated with sintilimab.
Keywords: Checkpoint inhibitor-related immune-mediated liver injury; Hepatotoxicity; Risk factors; Sintilimab; Updated RUCAM.
© 2023 Authors.
Conflict of interest statement
The authors have no conflict of interests related to this publication.
Figures
Similar articles
-
Advances in Idiosyncratic Drug-Induced Liver Injury Issues: New Clinical and Mechanistic Analysis Due to Roussel Uclaf Causality Assessment Method Use.Int J Mol Sci. 2023 Jun 29;24(13):10855. doi: 10.3390/ijms241310855. Int J Mol Sci. 2023. PMID: 37446036 Free PMC article. Review.
-
Utility of the Roussel Uclaf Causality Assessment Method (RUCAM) to analyze the hepatic findings in a clinical trial program: evaluation of the direct thrombin inhibitor ximelagatran.Int J Clin Pharmacol Ther. 2008 Jul;46(7):327-39. doi: 10.5414/cpp46327. Int J Clin Pharmacol Ther. 2008. PMID: 18793587
-
Severe tyrosine-kinase inhibitor induced liver injury in metastatic renal cell carcinoma patients: two case reports assessed for causality using the updated RUCAM and review of the literature.BMC Gastroenterol. 2022 Feb 5;22(1):49. doi: 10.1186/s12876-022-02121-3. BMC Gastroenterol. 2022. PMID: 35123392 Free PMC article. Review.
-
Development and validation of a nomogram for predicting immune-related pneumonitis after sintilimab treatment.Cancer Med. 2024 Feb;13(3):e6708. doi: 10.1002/cam4.6708. Epub 2024 Jan 12. Cancer Med. 2024. PMID: 38214102 Free PMC article.
-
Androgenic anabolic steroid-induced liver injury: two case reports assessed for causality by the updated Roussel Uclaf Causality Assessment Method (RUCAM) score and a comprehensive review of the literature.BMJ Open Gastroenterol. 2020 Nov;7(1):e000549. doi: 10.1136/bmjgast-2020-000549. BMJ Open Gastroenterol. 2020. PMID: 33214235 Free PMC article. Review.
Cited by
-
The effectiveness and safety of posaconazole enteric-coated tablet versus oral suspension in invasive fungal infections.Sci Rep. 2024 Nov 13;14(1):27887. doi: 10.1038/s41598-024-79512-x. Sci Rep. 2024. PMID: 39538016 Free PMC article.
-
Risk Factors of Immune-Mediated Hepatotoxicity Induced by Immune Checkpoint Inhibitors in Cancer Patients: A Systematic Review and Meta-Analysis.Curr Oncol. 2024 Nov 13;31(11):7129-7143. doi: 10.3390/curroncol31110525. Curr Oncol. 2024. PMID: 39590156 Free PMC article.
-
Drug Induced Liver Injury: Highlights and Controversies in the 2023 Literature.Drug Saf. 2025 May;48(5):455-488. doi: 10.1007/s40264-025-01514-z. Epub 2025 Feb 8. Drug Saf. 2025. PMID: 39921708 Review.
References
-
- Kaehler KC, Piel S, Livingstone E, Schilling B, Hauschild A, Schadendorf D. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol. 2010;37(5):485–498. doi: 10.1053/j.seminoncol.2010.09.003. - DOI - PubMed
-
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
