Association of gender with cardiovascular and autonomic responses to central hypovolemia
- PMID: 37719984
- PMCID: PMC10501725
- DOI: 10.3389/fcvm.2023.1211774
Association of gender with cardiovascular and autonomic responses to central hypovolemia
Abstract
Introduction: Lower body negative pressure (LBNP) eliminates the impact of weight-bearing muscles on venous return, as well as the vestibular component of cardiovascular and autonomic responses. We evaluated the hemodynamic and autonomic responses to central hypovolemia, induced by LBNP in both males and females.
Methodology: A total of 44 participants recruited in the study. However, 9 participants did not complete the study protocol. Data from the remaining 35 participants were analysed, 18 males (25.28 ± 3.61 years, 181.50 ± 7.43 cm height, 74.22 ± 9.16 kg weight) and 17 females (22.41 ± 2.73 years, 167.41 ± 6.29 cm height, 59.06 ± 6.91 kg weight). During the experimental protocol, participants underwent three phases, which included 30 min of supine rest, four 4 min intervals of stepwise increases in LBNP from -10 mmHg to -40 mmHg, and 5 min of supine recovery. Throughout the protocol, hemodynamic variables such as blood pressure, heart rate, stroke index, cardiac index, and total peripheral resistance index were continuously monitored. Autonomic variables were calculated from heart rate variability measures, using low and high-frequency spectra, as indicators of sympathetic and parasympathetic activity, respectively.
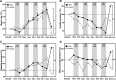
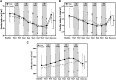
Results: At rest, males exhibited higher systolic (118.56 ± 9.59 mmHg and 110.03 ± 10.88 mmHg, p < 0.05) and mean arterial (89.70 ± 6.86 and 82.65 ± 9.78, p < 0.05) blood pressure as compared to females. Different levels of LBNP altered hemodynamic variables in both males and females: heart rate [F(1,16) = 677.46, p < 0.001], [F(1,16) = 550.87, p < 0.001]; systolic blood pressures [F(1,14) = 3,186.77, p < 0.001], [F(1,17) = 1,345.61, p < 0.001]; diastolic blood pressure [F(1,16) = 1,669.458, p < 0.001], [F(1,16) = 1,127.656, p < 0.001]; mean arterial pressures [F(1,16) = 2,330.44, p < 0.001], [F(1,16) = 1,815.68, p < 0.001], respectively. The increment in heart rates during LBNP was significantly different between both males and females (p = 0.025). The low and high-frequency powers were significantly different for males and females (p = 0.002 and p = 0.001, respectively), with the females having a higher increase in low-frequency spectral power.
Conclusions and future directions: Cardiovascular activity and autonomic function at rest are influenced by gender. During LBNP application, hemodynamic and autonomic responses differed between genders. These gender-based differences in responses during central hypovolemia could potentially be attributed to the lower sympathetic activity in females. With an increasing number of female crew members in space missions, it is important to understand the role sex-steroid hormones play in the regulation of cardiovascular and autonomic activity, at rest and during LBNP.
Keywords: autonomic; gender; heart rate variability; hemodynamics; lower body negative pressure; sex; sympathetic; vascular.
© 2023 Shankhwar, Urvec, Steuber, Schmid Zalaudek, Bergauer, Alsuwaidi, Du Plessis, Alsheikh-Ali, Kellett, Bayoumi, Blaber and Goswami.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Schlabs T, Rosales-velderrain A, Ruckstuhl H, Stahn AC, Hargens AR. Comparison of cardiovascular and biomechanical parameters of supine lower body negative pressure and upright lower body positive pressure to simulate activity in 1/6G and 3/8G. J Appl Physiol. (2013) 115:275–84. 10.1152/japplphysiol.00990.2012 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

