Bifidobacterium affects antitumor efficacy of oncolytic adenovirus in a mouse model of melanoma
- PMID: 37720092
- PMCID: PMC10502363
- DOI: 10.1016/j.isci.2023.107668
Bifidobacterium affects antitumor efficacy of oncolytic adenovirus in a mouse model of melanoma
Abstract
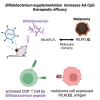
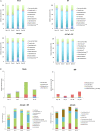
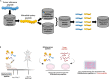
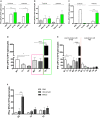
Gut microbiota plays a key role in modulating responses to cancer immunotherapy in melanoma patients. Oncolytic viruses (OVs) represent emerging tools in cancer therapy, inducing a potent immunogenic cancer cell death (ICD) and recruiting immune cells in tumors, poorly infiltrated by T cells. We investigated whether the antitumoral activity of oncolytic adenovirus Ad5D24-CpG (Ad-CpG) was gut microbiota-mediated in a syngeneic mouse model of melanoma and observed that ICD was weakened by vancomycin-mediated perturbation of gut microbiota. Ad-CpG efficacy was increased by oral supplementation with Bifidobacterium, reducing melanoma progression and tumor-infiltrating regulatory T cells. Fecal microbiota was enriched in bacterial species belonging to the Firmicutes phylum in mice treated with both Bifidobacterium and Ad-CpG; furthermore, our data suggest that molecular mimicry between melanoma and Bifidobacterium-derived epitopes may favor activation of cross-reactive T cells and constitutes one of the mechanisms by which gut microbiota modulates OVs response.
Keywords: Cancer; Microbiome; Virology.
© 2023 The Authors.
Conflict of interest statement
Vincenzo Cerullo is founder and shareholder at VALO therapeutics.
Figures




References
LinkOut - more resources
Full Text Sources

