Highly sensitive visual restoration and protection via ectopic expression of chimeric rhodopsin in mice
- PMID: 37720108
- PMCID: PMC10504486
- DOI: 10.1016/j.isci.2023.107716
Highly sensitive visual restoration and protection via ectopic expression of chimeric rhodopsin in mice
Abstract
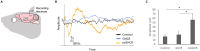
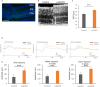
Photoreception requires amplification by mammalian rhodopsin through G protein activation, which requires a visual cycle. To achieve this in retinal gene therapy, we incorporated human rhodopsin cytoplasmic loops into Gloeobacter rhodopsin, thereby generating Gloeobacter and human chimeric rhodopsin (GHCR). In a murine model of inherited retinal degeneration, we induced retinal GHCR expression by intravitreal injection of a recombinant adeno-associated virus vector. Retinal explant and visual thalamus electrophysiological recordings, behavioral tests, and histological analysis showed that GHCR restored dim-environment vision and prevented the progression of retinal degeneration. Thus, GHCR may be a potent clinical tool for the treatment of retinal disorders.
Keywords: Behavioral neuroscience; Molecular neuroscience; Neurology.
© 2023 The Author(s).
Conflict of interest statement
Y.K., H.K., K.T., and T.K. are inventors on pending patents (PCT/JP2017/031579、PCT/JP2019/1565) related to this work. Y.K. is CEO of Restore Vision Inc.
Figures




References
-
- Doroudchi M.M., Greenberg K.P., Liu J., Silka K.A., Boyden E.S., Lockridge J.A., Arman A.C., Janani R., Boye S.E., Boye S.L., et al. Virally delivered Channelrhodopsin-2 Safely and Effectively Restores Visual Function in Multiple Mouse Models of Blindness. Mol. Ther. 2011;19:1220–1229. doi: 10.1038/MT.2011.69. - DOI - PMC - PubMed
-
- Cronin T., Vandenberghe L.H., Hantz P., Juttner J., Reimann A., Kacsó A.E., Huckfeldt R.M., Busskamp V., Kohler H., Lagali P.S., et al. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol. Med. 2014;6:1175–1190. doi: 10.15252/emmm.201404077. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

