Microbe-host interactions: structure and functions of Gram-negative bacterial membrane vesicles
- PMID: 37720140
- PMCID: PMC10500606
- DOI: 10.3389/fmicb.2023.1225513
Microbe-host interactions: structure and functions of Gram-negative bacterial membrane vesicles
Abstract
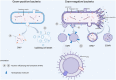
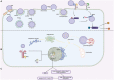
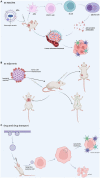
Bacteria-host interaction is a common, relevant, and intriguing biological phenomena. The host reacts actively or passively to the bacteria themselves, their products, debris, and so on, through various defense systems containing the immune system, the bacteria communicate with the local or distal tissues of the host via their own surface antigens, secreted products, nucleic acids, etc., resulting in relationships of attack and defense, adaptation, symbiosis, and even collaboration. The significance of bacterial membrane vesicles (MVs) as a powerful vehicle for the crosstalk mechanism between the two is growing. In the recent decade, the emergence of MVs in microbial interactions and a variety of bacterial infections, with multiple adhesions to host tissues, cell invasion and evasion of host defense mechanisms, have brought MVs to the forefront of bacterial pathogenesis research. Whereas MVs are a complex combination of molecules not yet fully understood, research into its effects, targeting and pathogenic components will advance its understanding and utilization. This review will summarize structural, extraction and penetration information on several classes of MVs and emphasize the role of MVs in transport and immune response activation. Finally, the potential of MVs as a therapeutic method will be highlighted, as will future research prospects.
Keywords: Gram-negative; application; immune response; interactions; membrane vesicles.
Copyright © 2023 Xiao, Li and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Agarkov N. M., Makaryan A. S., Gontareva I. S. (2020). Advancing diagnostics of chronic paradontitis in children. Russian J. Infect. Immun. 10, 558–564. doi: 10.15789/2220-7619-ADO-1186 - DOI
-
- Ahmadi Badi S., Khatami S. H., Irani S. H., Siadat S. D. (2019). InductioneEffects of bacteroides fragilis derived outer membrane vesicles on toll like receptor 2, toll like receptor 4 genes expression and cytokines concentration in human intestinal epithelial cells. Cell J. 21, 57–61. doi: 10.22074/cellj.2019.5750, PMID: - DOI - PMC - PubMed
-
- Alaniz R. C., Deatherage B. L., Lara J. C., Cookson B. T. (2007). Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 179, 7692–7701. doi: 10.4049/jimmunol.179.11.7692, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

