Sporulation conditions influence the surface and adhesion properties of Bacillus subtilis spores
- PMID: 37720141
- PMCID: PMC10502511
- DOI: 10.3389/fmicb.2023.1219581
Sporulation conditions influence the surface and adhesion properties of Bacillus subtilis spores
Abstract
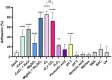
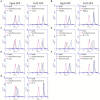
Spore-forming bacteria of the Bacillus subtilis group are responsible for recurrent contamination of processing lines in the food industry which can lead to food spoilage. The persistence of B. subtilis would be due to the high resistance of spores to extreme environmental condition and their propensity to contaminate surfaces. While it is well known that sporulation conditions modulate spore resistance properties, little is known about their effect on surface and adhesion properties. Here, we studied the impact of 13 sporulation conditions on the surface and adhesion properties of B. subtilis 168 spores. We showed that Ca2+ or Mg2+ depletion, lower oxygen availability, acidic pH as well as oxidative stresses during sporulation lead to the release of more hydrophobic and adherent spores. The consequences of these sporulation conditions on crust composition in carbohydrates and proteins were also evaluated. The crust glycans of spores produced in a sporulation medium depleted in Ca2+ or Mg2+ or oxygen-limited conditions were impaired and contained lower amounts of rhamnose and legionaminic acid. In addition, we showed that lower oxygen availability or addition of hydrogen peroxide during sporulation decreases the relative amount of two crust proteins (CgeA and CotY) and the changes observed in these conditions could be due to transcriptional repression of genes involved in crust synthesis in late stationary phase. The fact that sporulation conditions affect the ease with which spores can contaminate surfaces could explain the frequent and recurrent presence of B. subtilis spores in food processing lines.
Keywords: Bacillus subtilis; adhesion; crust; glycans; hydrophobicity; spores; sporulation.
Copyright © 2023 Hamiot, Lemy, Krzewinski, Faille and Dubois.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abe K., Kawano Y., Iwamoto K., Arai K., Maruyama Y., Eichenberger P., et al. . (2014). Developmentally-regulated excision of the SPβ prophage reconstitutes a gene required for spore envelope maturation in Bacillus subtilis. PLoS Genet. 10:e1004636. doi: 10.1371/journal.pgen.1004636, PMID: - DOI - PMC - PubMed
-
- Abe K., Takamatsu T., Sato T. (2017). Mechanism of bacterial gene rearrangement: SprA-catalyzed precise DNA recombination and its directionality control by SprB ensure the gene rearrangement and stable expression of spsM during sporulation in Bacillus subtilis. Nucleic Acids Res. 45, 6669–6683. doi: 10.1093/nar/gkx466, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

