Patterns of asexual reproduction of the soybean aphid, Aphis glycines (Matsumura), with and without the secondary symbionts Wolbachia and Arsenophonus, on susceptible and resistant soybean genotypes
- PMID: 37720159
- PMCID: PMC10501154
- DOI: 10.3389/fmicb.2023.1209595
Patterns of asexual reproduction of the soybean aphid, Aphis glycines (Matsumura), with and without the secondary symbionts Wolbachia and Arsenophonus, on susceptible and resistant soybean genotypes
Abstract
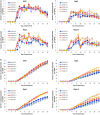
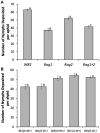
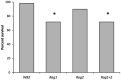
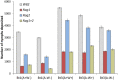
Plant breeding is used to develop crops with host resistance to aphids, however, virulent biotypes often develop that overcome host resistance genes. We tested whether the symbionts, Arsenophonus (A) and Wolbachia (W), affect virulence and fecundity in soybean aphid biotypes Bt1 and Bt3 cultured on whole plants and detached leaves of three resistant, Rag1, Rag2 and Rag1 + 2, and one susceptible, W82, soybean genotypes. Whole plants and individual aphid experiments of A. glycines with and without Arsenophonus and Wolbachia did not show differences in overall fecundity. Differences were observed in peak fecundity, first day of deposition, and day of maximum nymph deposition of individual aphids on detached leaves. Bt3 had higher fecundity than Bt1 on detached leaves of all plant genotypes regardless of bacterial profile. Symbionts did not affect peak fecundity of Bt1 but increased it in Bt3 (A+W+) and all Bt3 strains began to deposit nymphs earlier than the Bt1 (A+W-). Arsenophonus in Bt1 delayed the first day of nymph deposition in comparison to aposymbiotic Bt1 except when reared on Rag1 + 2. For the Bt1 and Bt3 strains, symbionts did not result in a significant difference in the day they deposited the maximum number of nymphs nor was there a difference in survival or variability in number of nymphs deposited. Variability of number of aphids deposited was higher in aphids feeding on resistant plant genotypes. The impact of Arsenophonus on soybean aphid patterns of fecundity was dependent on the aphid biotype and plant genotype. Wolbachia alone had no detectable impact but may have contributed to the increased fecundity of Bt3 (A+W+). An individual based model, using data from the detached leaves experiment and with intraspecific competition removed, found patterns similar to those observed in the greenhouse and growth chamber experiments including a significant interaction between soybean genotype and aphid strain. Combining individual data with the individual based model of population growth isolated the impact of fecundity and host resistance from intraspecific competition and host health. Changes to patterns of fecundity, influenced by the composition and concentration of symbionts, may contribute to competitive interactions among aphid genotypes and influence selection on virulent aphid populations.
Keywords: Arsenophonus; Hamiltonella; Wolbachia; reproduction; resistant soybean varieties; soybean aphid; symbionts.
Copyright © 2023 Giordano, Weber, Mitacek, Flores, Ledesma, De, Herman, Soto-Adames, Nguyen, Hill and Hartman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
The Endosymbiont Arsenophonus Provides a General Benefit to Soybean Aphid (Hemiptera: Aphididae) Regardless of Host Plant Resistance (Rag).Environ Entomol. 2015 Jun;44(3):574-81. doi: 10.1093/ee/nvv031. Epub 2015 Mar 31. Environ Entomol. 2015. PMID: 26313962
-
Detached leaf and whole plant assays for soybean aphid resistance: differential responses among resistance sources and biotypes.J Econ Entomol. 2010 Jun;103(3):949-57. doi: 10.1603/ec09337. J Econ Entomol. 2010. PMID: 20568642
-
Establishment of in vitro soybean aphids, Aphis glycines (Hemiptera: Aphididae): a tool to facilitate studies of aphid symbionts, plant-insect interactions and insecticide efficacy.Pest Manag Sci. 2017 Jun;73(6):1229-1235. doi: 10.1002/ps.4448. Epub 2016 Dec 16. Pest Manag Sci. 2017. PMID: 27680689
-
The Resistant Soybean-Aphis glycines Interaction: Current Knowledge and Prospects.Front Plant Sci. 2020 Aug 11;11:1223. doi: 10.3389/fpls.2020.01223. eCollection 2020. Front Plant Sci. 2020. PMID: 32849757 Free PMC article. Review.
-
Rapid evolution to host plant resistance by an invasive herbivore: soybean aphid (Aphis glycines) virulence in North America to aphid resistant cultivars.Curr Opin Insect Sci. 2018 Apr;26:1-7. doi: 10.1016/j.cois.2017.12.006. Epub 2018 Jan 5. Curr Opin Insect Sci. 2018. PMID: 29764648 Review.
References
-
- Agresti A. (2002). Categorical data analysis. Wiley Series in Probability and Statistics. Hoboken, NJ: John Wiley & Sons.
-
- Alt J., Ryan-Mahmutagic M. (2013). Soybean aphid biotype 4 identified. Crop Sci. 53, 1491–1495. doi: 10.2135/cropsci2012.11.0672 - DOI
-
- Bai X., Zhang W., Orantes L., Jun T.-H., Mittapalli O., Mian M. A. R., et al. . (2010). Combining next-generation sequencing strategies for rapid molecular resource development from an invasive aphid species, Aphis glycines. PLoS ONE 5:e11370. doi: 10.1371/journal.pone.0011370, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

