Efficacy and Safety of Anlotinib-Containing Regimens in Advanced Non-Small Cell Lung Cancer: A Real-World Study
- PMID: 37720175
- PMCID: PMC10505018
- DOI: 10.2147/IJGM.S424777
Efficacy and Safety of Anlotinib-Containing Regimens in Advanced Non-Small Cell Lung Cancer: A Real-World Study
Abstract
Purpose: Anlotinib is widely used in the clinical treatment of non-small cell lung cancer (NSCLC), alone or in combination with other anticancer drugs. The aim of this study was to investigate the real-world efficacy and safety of anlotinib-containing regimens.
Patients and methods: Confirmed advanced NSCLC patients who had received anlotinib alone or in combination were enrolled. An overall analysis of the efficacy and safety of anlotinib was performed in all patients, and then subgroup analysis was used to further compare the efficacy between anlotinib monotherapy and combination therapy. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were ADR, ORR, and DCR.
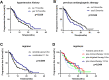
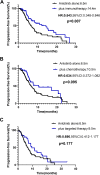
Results: A total of 240 patients were included. The overall median PFS was 8.5 months (95% confidence interval [CI]: 7.1-9.9 months). Anlotinib treatment regimens (monotherapy or combination therapy) and whether they received previous antiangiogenesis were associated with PFS. Anlotinib plus immunotherapy achieved longer PFS than anlotinib monotherapy (median PFS: 10.5 vs 6.5 months, p=0.007). Stratification analysis showed the PFS of anlotinib plus immunotherapy was significantly longer in male, adenocarcinoma, <=65 years old, patients stage IV, EGFR wild type, with extrathoracic metastasis, performance status scores ≥2, the first-line treatment, patients with a history of hypertension and no previous antiangiogenesis than anlotinib monotherapy. The median PFS of anlotinib plus chemotherapy, targeted therapy was slightly longer than anlotinib alone (respectively, 10.5 vs 6.5 months, p=0.095; 9.5 vs 6.5 months, p=0.177). Adverse reactions were mostly mild and acceptable, with hypertension being the most common.
Conclusion: Anlotinib is effective and tolerable in advanced NSCLC patients. Immunotherapy combination with anlotinib significantly improved PFS. The efficacy of anlotinib may be impaired by previous antiangiogenic therapy, which can be investigated in further studies.
Keywords: anlotinib; combination therapy; efficacy; immunotherapy; non-small cell lung cancer; safety.
© 2023 Sun et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

