Targeting neoantigens to APC-surface molecules improves the immunogenicity and anti-tumor efficacy of a DNA cancer vaccine
- PMID: 37720215
- PMCID: PMC10499626
- DOI: 10.3389/fimmu.2023.1234912
Targeting neoantigens to APC-surface molecules improves the immunogenicity and anti-tumor efficacy of a DNA cancer vaccine
Erratum in
-
Corrigendum: Targeting neoantigens to APC-surface molecules improves the immunogenicity and anti-tumor efficacy of a DNA cancer vaccine.Front Immunol. 2023 Sep 14;14:1290431. doi: 10.3389/fimmu.2023.1290431. eCollection 2023. Front Immunol. 2023. PMID: 37781410 Free PMC article.
Abstract
Introduction: Tumor-specific mutations generate neoepitopes unique to the cancer that can be recognized by the immune system, making them appealing targets for therapeutic cancer vaccines. Since the vast majority of tumor mutations are patient-specific, it is crucial for cancer vaccine designs to be compatible with individualized treatment strategies. Plasmid DNA vaccines have substantiated the immunogenicity and tumor eradication capacity of cancer neoepitopes in preclinical models. Moreover, early clinical trials evaluating personalized neoepitope vaccines have indicated favorable safety profiles and demonstrated their ability to elicit specific immune responses toward the vaccine neoepitopes.
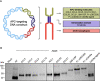
Methods: By fusing in silico predicted neoepitopes to molecules with affinity for receptors on the surface of APCs, such as chemokine (C-C motif) ligand 19 (CCL19), we designed an APC-targeting cancer vaccine and evaluated their ability to induce T-cell responses and anti-tumor efficacy in the BALB/c syngeneic preclinical tumor model.
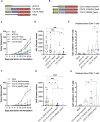
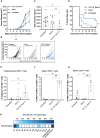
Results: In this study, we demonstrate how the addition of an antigen-presenting cell (APC) binding molecule to DNA-encoded cancer neoepitopes improves neoepitope-specific T-cell responses and the anti-tumor efficacy of plasmid DNA vaccines. Dose-response evaluation and longitudinal analysis of neoepitope-specific T-cell responses indicate that combining APC-binding molecules with the delivery of personalized tumor antigens holds the potential to improve the clinical efficacy of therapeutic DNA cancer vaccines.
Discussion: Our findings indicate the potential of the APC-targeting strategy to enhance personalized DNA cancer vaccines while acknowledging the need for further research to investigate its molecular mechanism of action and to translate the preclinical results into effective treatments for cancer patients.
Keywords: APC-targeting; CCL19; DNA vaccine; cancer immunotherapy; neoantigens.
Copyright © 2023 Barrio-Calvo, Kofoed, Holste, Sørensen, Viborg, Kringelum, Kleine-Kohlbrecher, Steenmans, Thygesen, Rønø and Friis.
Conflict of interest statement
MB-C, SK, SE, NV, AS, JK, DK-K, CS, CT, BR, and SF are employed at Evaxion Biotech A/S. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Mørk SK, Kadivar M, Bol KF, Draghi A, Westergaard MCW, Skadborg SK, et al. . Personalized therapy with peptide-based neoantigen vaccine (EVX-01) including a novel adjuvant, CAF®09b, in patients with metastatic melanoma. Oncoimmunology (2022) 11:2023255. doi: 10.1080/2162402X.2021.2023255 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

