Escherichia coli adhesion portion FimH polarizes M2 macrophages to M1 macrophages in tumor microenvironment via toll-like receptor 4
- PMID: 37720226
- PMCID: PMC10502728
- DOI: 10.3389/fimmu.2023.1213467
Escherichia coli adhesion portion FimH polarizes M2 macrophages to M1 macrophages in tumor microenvironment via toll-like receptor 4
Abstract
Background: Macrophages are key effector cells of innate immunity and play a critical role in the immune balance of disease pathogenesis, especially in the tumor microenvironment. In previous studies, we showed that FimH, an Escherichia coli adhesion portion, promoted dendritic cell activation. However, the effect of FimH in macrophage polarization has yet to be fully examined. In this study, we investigated the potential effect of FimH on macrophages, as well as the polarization from M2 to M1 macrophages, contributing to the overall antitumor effect.
Methods: Mouse bone marrow derived macrophages and peritoneal macrophages were generated to test the effect of FimH in vitro. The expression of costimulatory molecules and production of cytokines were analyzed. The effect of FimH in the tumor-associated macrophages was examine in the B16F10-tumor bearing C57BL/6.
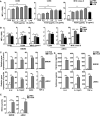
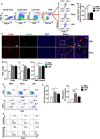
Results: FimH was found to promote M1 macrophage activation. In addition, FimH polarized M2 macrophages, which were induced by interleukin (IL)-4 and IL-13 into M1 macrophages were dependent on toll-like receptor 4 and myeloid differentiation factor 2. Moreover, FimH reprogramed the tumor-associated macrophage (TAM) into M1 macrophages in B16 melanoma tumor-bearing mice and promoted an inflammatory reaction in the tumor microenvironment (TME). Furthermore, FimH promoted M1 macrophage activation, as well as the reversion of M2 macrophages into M1 macrophages in humans. Finally, FimH treatment was found to enhance the anti-cancer immunity of anti-PD-L1 antibody by the induction of M1 polarization from TAM.
Conclusion: This study demonstrated the potential effect of FimH on the activation of macrophages, responsible for the repolarization of M2 macrophages into the M1 phenotype via the TLR4 signaling pathway. Moreover, FimH could also reprogram TAM polarization to the M1 status in the TME, as well as enhance the anti-tumor activity of immune checkpoint blockade.
Keywords: fimH; macrophage polarization; myeloid differentiation factor 2; toll-like receptor 4; tumor-associated macrophage.
Copyright © 2023 Zhang, Xu, Zhang, Xu and Jin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

