Internalization of extracellular vesicles from Lactobacillus johnsonii N6.2 elicit an RNA sensory response in human pancreatic cell lines
- PMID: 37720361
- PMCID: PMC10500552
- DOI: 10.1002/jex2.101
Internalization of extracellular vesicles from Lactobacillus johnsonii N6.2 elicit an RNA sensory response in human pancreatic cell lines
Abstract
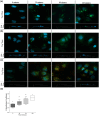
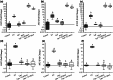
Cells of all domains of life can secrete extracellular vesicles (EV). These secreted vesicles have been indicated as vehicles carrying molecules that facilitate intra- and inter-species interaction. Lactobacillus johnsonii N6.2, a bacterium used in probiotic preparations, has been shown to produce nano-sized EV. In the present work we used L. johnsonii N6.2 EV, concentrated from exosome depleted MRS supernatant, to identify the uptake mechanisms of EV and the impact of the RNA cargo in the EV on the upregulation of the cellular response of βlox5 human pancreatic cells. Using eukaryotic uptake inhibitors, it was found that EV are internalized by the clathrin/dynamin mediated endocytosis pathway. Further co-localization experiments with the endosome markers RAB5, RAB7 and LAMP1 as well as calcein indicated that EV escape the endosome shortly after RAB7 fusion. Using the expression of the 2',5'-oligoadenylate synthetase (OAS) host pathway, previously identified as targeted by L. johnsonii EV, we found that the host cellular response to the EV are dependent on the integrity of the external components of the EV as well as on the RNA cargo. Global transcriptome analysis was performed on EV and the bacterial whole cell. It was found that the RNA transcripts found within the EV largely represent the most abundantly transcribed genes in the bacterial cells such as those associated with protein synthesis and glycolysis. Further analysis showed an enrichment of smaller size transcripts as well as those encoding for membrane bound or extracellular proteins in L. johnsonii's EV.
Keywords: 2’−5’-oligoadenylate synthetase; Lactobacillus johnsonii N6.2; endosome; extracellular vesicles; human beta cell line; nanovesicles; probiotic; βlox5.
Conflict of interest statement
Declaration of Interest Statement Dr. Graciela Lorca holds U.S. patent No. 9,474,773 and 9,987,313 on Lactobacillus johnsonii N6.2. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures


Similar articles
-
Identification of Biomarkers for Systemic Distribution of Nanovesicles From Lactobacillus johnsonii N6.2.Front Immunol. 2021 Aug 31;12:723433. doi: 10.3389/fimmu.2021.723433. eCollection 2021. Front Immunol. 2021. PMID: 34531870 Free PMC article.
-
The Sdp-SH3b2 domain contained in Lactobacillus johnsonii N6.2-derived extracellular vesicles inhibit murine norovirus replication.Front Immunol. 2024 Dec 5;15:1490755. doi: 10.3389/fimmu.2024.1490755. eCollection 2024. Front Immunol. 2024. PMID: 39712028 Free PMC article.
-
Bile promotes Lactobacillus johnsonii N6.2 extracellular vesicle production with conserved immunomodulatory properties.Sci Rep. 2024 May 28;14(1):12272. doi: 10.1038/s41598-024-62843-0. Sci Rep. 2024. PMID: 38806562 Free PMC article.
-
Uptake and Fate of Extracellular Membrane Vesicles: Nucleoplasmic Reticulum-Associated Late Endosomes as a New Gate to Intercellular Communication.Cells. 2020 Aug 21;9(9):1931. doi: 10.3390/cells9091931. Cells. 2020. PMID: 32825578 Free PMC article. Review.
-
Exosomes provide unappreciated carrier effects that assist transfers of their miRNAs to targeted cells; I. They are 'The Elephant in the Room'.RNA Biol. 2021 Nov;18(11):2038-2053. doi: 10.1080/15476286.2021.1885189. Epub 2021 May 4. RNA Biol. 2021. PMID: 33944671 Free PMC article. Review.
Cited by
-
Lactobacillus johnsonii N6.2 Phospholipids Induce T Cell Anergy upon Cognate Dendritic Cell Interactions.Metabolites. 2025 Apr 22;15(5):284. doi: 10.3390/metabo15050284. Metabolites. 2025. PMID: 40422862 Free PMC article.
-
Ligilactobacillus-Derived Extracellular Vesicles Inhibit Growth and Virulence of Enteric Pathogens.Probiotics Antimicrob Proteins. 2024 Dec 16. doi: 10.1007/s12602-024-10423-z. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 39680344
-
Erucic acid utilization by Lactobacillus johnsonii N6.2.Front Microbiol. 2024 Nov 25;15:1476958. doi: 10.3389/fmicb.2024.1476958. eCollection 2024. Front Microbiol. 2024. PMID: 39654680 Free PMC article.
-
Potential Effects of Hyperglycemia on SARS-CoV-2 Entry Mechanisms in Pancreatic Beta Cells.Viruses. 2024 Aug 2;16(8):1243. doi: 10.3390/v16081243. Viruses. 2024. PMID: 39205219 Free PMC article. Review.
-
Microbial Extracellular Vesicles in Host-Microbiota Interactions.Results Probl Cell Differ. 2024;73:475-520. doi: 10.1007/978-3-031-62036-2_19. Results Probl Cell Differ. 2024. PMID: 39242390 Review.
References
-
- Busse, D. C. , Habgood‐Coote, D. , Clare, S. , Brandt, C. , Bassano, I. , Kaforou, M. , Herberg, J. , Levin, M. , Eléouët, J. F. , Kellam, P. , & Tregoning, J. S. (2020). Interferon‐induced protein 44 and interferon‐induced protein 44‐like restrict replication of respiratory syncytial virus. Journal of Virology, 94(18), 10–1128. 10.1128/jvi.00297-20 - DOI - PMC - PubMed
-
- Cañas, M. A. , Giménez, R. , Fábrega, M. J. , Toloza, L. , Baldomà, L. , & Badia, J. (2016). Outer membrane vesicles from the probiotic Escherichia coli Nissle 1917 and the commensal ECOR12 enter intestinal epithelial cells via clathrin‐dependent endocytosis and elicit differential effects on DNA damage. PLoS ONE, 11(8), e0160374. 10.1371/journal.pone.0160374 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
