AQP4-independent TRPV4 modulation of plasma membrane water permeability
- PMID: 37720545
- PMCID: PMC10500071
- DOI: 10.3389/fncel.2023.1247761
AQP4-independent TRPV4 modulation of plasma membrane water permeability
Abstract
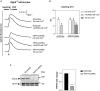
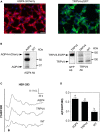
Despite of the major role of aquaporin (AQP) water channels in controlling transmembrane water fluxes, alternative ways for modulating water permeation have been proposed. In the Central Nervous System (CNS), Aquaporin-4 (AQP4) is reported to be functionally coupled with the calcium-channel Transient-Receptor Potential Vanilloid member-4 (TRPV4), which is controversially involved in cell volume regulation mechanisms and water transport dynamics. The present work aims to investigate the selective role of TRPV4 in regulating plasma membrane water permeability in an AQP4-independent way. Fluorescence-quenching water transport experiments in Aqp4-/- astrocytes revealed that cell swelling rate is significantly increased upon TRPV4 activation and in the absence of AQP4. The biophysical properties of TRPV4-dependent water transport were therefore assessed using the HEK-293 cell model. Calcein quenching experiments showed that chemical and thermal activation of TRPV4 overexpressed in HEK-293 cells leads to faster swelling kinetics. Stopped-flow light scattering water transport assay was used to measure the osmotic permeability coefficient (Pf, cm/s) and activation energy (Ea, kcal/mol) conferred by TRPV4. Results provided evidence that although the Pf measured upon TRPV4 activation is lower than the one obtained in AQP4-overexpressing cells (Pf of AQP4 = 0.01667 ± 0.0007; Pf of TRPV4 = 0.002261 ± 0.0004; Pf of TRPV4 + 4αPDD = 0.007985 ± 0.0006; Pf of WT = 0.002249 ± 0.0002), along with activation energy values (Ea of AQP4 = 0.86 ± 0.0006; Ea of TRPV4 + 4αPDD = 2.73 ± 1.9; Ea of WT = 8.532 ± 0.4), these parameters were compatible with a facilitated pathway for water movement rather than simple diffusion. The possibility to tune plasma membrane water permeability more finely through TRPV4 might represent a protective mechanism in cells constantly facing severe osmotic challenges to avoid the potential deleterious effects of the rapid cell swelling occurring via AQP channels.
Keywords: AQP4; TRPV4; astrocytes; calcium ions; cell swelling; ion channels; water channels.
Copyright © 2023 Barile, Mola, Formaggio, Saracino, Cibelli, Gargano, Mogni, Frigeri, Caprini, Benfenati and Nicchia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Ahmadi D., Thompson K. C., García Sakai V., Schweins R., Moulin M., Haertlein M., et al. (2022). Nanoscale structure and dynamics of model membrane lipid raft systems, studied by neutron scattering methods. Front. Phys. 10:326. 10.3389/FPHY.2022.864746/BIBTEX - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

