Phase separation and pathologic transitions of RNP condensates in neurons: implications for amyotrophic lateral sclerosis, frontotemporal dementia and other neurodegenerative disorders
- PMID: 37720552
- PMCID: PMC10502346
- DOI: 10.3389/fnmol.2023.1242925
Phase separation and pathologic transitions of RNP condensates in neurons: implications for amyotrophic lateral sclerosis, frontotemporal dementia and other neurodegenerative disorders
Abstract
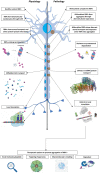
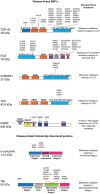
Liquid-liquid phase separation results in the formation of dynamic biomolecular condensates, also known as membrane-less organelles, that allow for the assembly of functional compartments and higher order structures within cells. Multivalent, reversible interactions between RNA-binding proteins (RBPs), including FUS, TDP-43, and hnRNPA1, and/or RNA (e.g., RBP-RBP, RBP-RNA, RNA-RNA), result in the formation of ribonucleoprotein (RNP) condensates, which are critical for RNA processing, mRNA transport, stability, stress granule assembly, and translation. Stress granules, neuronal transport granules, and processing bodies are examples of cytoplasmic RNP condensates, while the nucleolus and Cajal bodies are representative nuclear RNP condensates. In neurons, RNP condensates promote long-range mRNA transport and local translation in the dendrites and axon, and are essential for spatiotemporal regulation of gene expression, axonal integrity and synaptic function. Mutations of RBPs and/or pathologic mislocalization and aggregation of RBPs are hallmarks of several neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), and Alzheimer's disease. ALS/FTD-linked mutations of RBPs alter the strength and reversibility of multivalent interactions with other RBPs and RNAs, resulting in aberrant phase transitions. These aberrant RNP condensates have detrimental functional consequences on mRNA stability, localization, and translation, and ultimately lead to compromised axonal integrity and synaptic function in disease. Pathogenic protein aggregation is dependent on various factors, and aberrant dynamically arrested RNP condensates may serve as an initial nucleation step for pathologic aggregate formation. Recent studies have focused on identifying mechanisms by which neurons resolve phase transitioned condensates to prevent the formation of pathogenic inclusions/aggregates. The present review focuses on the phase separation of neurodegenerative disease-linked RBPs, physiological functions of RNP condensates, and the pathologic role of aberrant phase transitions in neurodegenerative disease, particularly ALS/FTD. We also examine cellular mechanisms that contribute to the resolution of aberrant condensates in neurons, and potential therapeutic approaches to resolve aberrantly phase transitioned condensates at a molecular level.
Keywords: RNA-binding proteins; aggregation; biomolecular condensates; neurodegenerative disease; phase separation.
Copyright © 2023 Naskar, Nayak, Salaikumaran, Vishal and Gopal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

