A Datasheet for the INSIGHT University Hospitals Birmingham Retinal Vein Occlusion Data Set
- PMID: 37720555
- PMCID: PMC10500462
- DOI: 10.1016/j.xops.2023.100388
A Datasheet for the INSIGHT University Hospitals Birmingham Retinal Vein Occlusion Data Set
Abstract
Purpose: Retinal vein occlusion (RVO) is the second leading cause of visual loss due to retinal disease. Retinal vein occlusion increases the risk of cardiovascular mortality and the risk of stroke. This article describes the data contained within the INSIGHT eye health data set for RVO and cardiovascular disease.
Design: Data set descriptor for routinely collected eye and systemic disease data.
Participants: All people who had suffered an RVO aged ≥ 18 years old, attending the Ophthalmology Clinic at Queen Elizabeth Hospital, University Hospitals Birmingham (UHB) National Health Service (NHS) Trust were included.
Methods: The INSIGHT Health Data Research Hub for Eye Health is an NHS-led ophthalmic bioresource. It provides researchers with safe access to anonymized routinely collected data from contributing NHS hospitals to advance research for patient benefit. This report describes the INSIGHT UHB RVO and major adverse cardiovascular events data set, a data set of ophthalmology and systemic data derived from the United Kingdom's largest acute care trust.
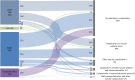
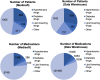
Main outcome measures: This data set consists of routinely collected data from the hospital's electronic patient records. The data set primarily includes structured data (relating to their hospital eye care and any cardiovascular data held for the individual) and OCT ocular images. Further details regarding the available data points are available in the supplementary information.
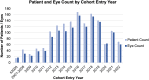
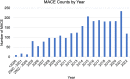
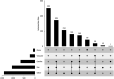
Results: At the time point of this analysis (September 30, 2022) the data set was composed of clinical data from 1521 patients, from Medisoft records inception. The data set includes 2196 occurrences of RVO affecting 2026 eyes, longitudinal eye follow-up clinical parameters, over 6217 eye-related procedures, and 982 encountered complications. The data set contains information on 2534 major adverse cardiovascular event occurrences, their subtype, number experienced per patient, and chronological relation to RVO event. Longitudinal follow-up data including laboratory results, regular medications, and all-cause mortality are also available within the data set.
Conclusions: This data set descriptor article summarizes the data set contents, the process of its curation, and potential uses. The data set is available through the structured application process that ensures research studies are for patient benefit. Further information regarding the data repository and contact details can be found at https://www.insight.hdrhub.org/.
Financial disclosures: Proprietary or commercial disclosure may be found after the references.
Keywords: Biomedical data; Data set; Major Cardiovascular events; Myocardial infarction; Retinal vein occlusion.
© 2023 by the American Academy of Ophthalmology.
Figures
References
-
- Gale R., Pikoula M., Lee A.Y., et al. Real world evidence on 5661 patients treated for macular oedema secondary to branch retinal vein occlusion with intravitreal anti-vascular endothelial growth factor, intravitreal dexamethasone or macular laser. Br J Ophthalmol. 2021;105:549–554. doi: 10.1136/bjophthalmol-2020-315836. - DOI - PMC - PubMed
-
- Gale R., Gill C., Pikoula M., et al. Multicentre study of 4626 patients assesses the effectiveness, safety and burden of two categories of treatments for central retinal vein occlusion: intravitreal anti-vascular endothelial growth factor injections and intravitreal Ozurdex injections. Br J Ophthalmol. 2021;105:1571–1576. doi: 10.1136/bjophthalmol-2020-317306. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

