Suppression of Tumor Cell Lactate-generating Signaling Pathways Eradicates Murine PTEN/p53-deficient Aggressive-variant Prostate Cancer via Macrophage Phagocytosis
- PMID: 37721526
- PMCID: PMC10841690
- DOI: 10.1158/1078-0432.CCR-23-1441
Suppression of Tumor Cell Lactate-generating Signaling Pathways Eradicates Murine PTEN/p53-deficient Aggressive-variant Prostate Cancer via Macrophage Phagocytosis
Abstract
Purpose: Phosphatase and tensin homolog (PTEN) loss-of-function/PI3K pathway hyperactivation is associated with poor therapeutic outcomes and immune checkpoint inhibitor resistance across multiple malignancies. Our prior studies in Pb-Cre;PTENfl/flTrp53fl/fl genetically engineered mice (GEM) with aggressive-variant prostate cancer (AVPC) demonstrated tumor growth control in 60% mice following androgen deprivation therapy/PI3K inhibitor (PI3Ki)/programmed cell death protein 1 (PD-1) antibody combination, via abrogating lactate cross-talk between cancer cells and tumor-associated macrophages (TAM), and suppression of histone lactylation (H3K18lac)/phagocytic activation within TAM. Here, we targeted immunometabolic mechanism(s) of PI3Ki resistance, with the goal of durable tumor control in AVPC.
Experimental design: Pb-Cre;PTENfl/flTrp53fl/fl GEM were treated with PI3Ki (copanlisib), MEK inhibitor (trametinib) or Porcupine inhibitor (LGK'974) singly or their combinations. MRI was used to monitor tumor kinetics and immune/proteomic profiling/ex vivo coculture mechanistic studies were performed on GEM tumors or corresponding tumor-derived cell lines.
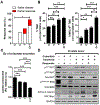
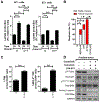
Results: Given our proteomic profiling showing persistent MEK signaling within tumors of PI3Ki-resistant GEM, we tested whether addition of trametinib to copanlisib enhances tumor control in GEM, and we observed 80% overall response rate via additive suppression of lactate within TME and H3K18lac within TAM, relative to copanlisib (37.5%) monotherapy. The 20% resistant mice demonstrated feedback Wnt/β-catenin activation, resulting in restoration of lactate secretion by tumor cells and H3K18lac within TAM. Cotargeting Wnt/β-catenin signaling with LGK'974 in combination with PI3Ki/MEKi, demonstrated durable tumor control in 100% mice via H3K18lac suppression and complete TAM activation.
Conclusions: Abrogation of lactate-mediated cross-talk between cancer cells and TAM results in durable ADT-independent tumor control in PTEN/p53-deficient AVPC, and warrants further investigation in clinical trials.
©2023 American Association for Cancer Research.
Conflict of interest statement
Dr. Patnaik has received research funding from Bristol Myers Squibb.
Figures



Update of
-
Suppression of tumor cell lactate-generating signaling pathways eradicates murine PTEN/p53-deficient aggressive-variant prostate cancer via macrophage phagocytosis.bioRxiv [Preprint]. 2023 May 23:2023.05.23.540590. doi: 10.1101/2023.05.23.540590. bioRxiv. 2023. Update in: Clin Cancer Res. 2023 Dec 1;29(23):4930-4940. doi: 10.1158/1078-0432.CCR-23-1441. PMID: 37292972 Free PMC article. Updated. Preprint.
References
-
- Li Y, Malapati S, Lin YT, Patnaik A. An integrative approach for sequencing therapies in metastatic prostate cancer. The American Journal of Hematology/Oncology 2017;13(12):26–31.
-
- Sydes MR, Spears MR, Mason MD, Clarke NW, Dearnaley DP, de Bono JS, et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol 2018;29(5):1235–48 doi 10.1093/annonc/mdy072. - DOI - PMC - PubMed
-
- Clarke NW, Ali A, Ingleby FC, Hoyle A, Amos CL, Attard G, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol 2019;30(12):1992–2003 doi 10.1093/annonc/mdz396. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

