Treatment Failure and Adverse Events After Amoxicillin-Clavulanate vs Amoxicillin for Pediatric Acute Sinusitis
- PMID: 37721610
- PMCID: PMC10509725
- DOI: 10.1001/jama.2023.15503
Treatment Failure and Adverse Events After Amoxicillin-Clavulanate vs Amoxicillin for Pediatric Acute Sinusitis
Erratum in
-
Error in Days for Outcome Ascertainment in Study Comparing Antibiotic Treatment of Acute Sinusitis in Children and Adolescents.JAMA. 2024 Sep 10;332(10):844-845. doi: 10.1001/jama.2024.16020. JAMA. 2024. PMID: 39159011 No abstract available.
-
Error in Study of Antibiotic Treatment for Acute Sinusitis in Children and Adolescents.JAMA. 2024 Sep 10;332(10):845. doi: 10.1001/jama.2024.15865. JAMA. 2024. PMID: 39159012 Free PMC article. No abstract available.
Abstract
Importance: Acute sinusitis is one of the most common indications for antibiotic prescribing in children, with an estimated 4.9 million such prescriptions in the US annually. Consensus does not exist regarding the optimal empirical antibiotic.
Objective: To compare amoxicillin-clavulanate vs amoxicillin for the treatment of acute sinusitis in outpatient children.
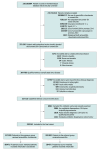
Design, setting, and participants: Cohort study of children and adolescents aged 17 years or younger with a new outpatient diagnosis of acute sinusitis and a same-day new prescription dispensation of amoxicillin-clavulanate or amoxicillin in a nationwide health care utilization database. Propensity score matching was used to mitigate confounding.
Exposure: A new prescription dispensation of amoxicillin-clavulanate or amoxicillin.
Main outcomes and measures: Treatment failure, defined as an aggregate of a new antibiotic dispensation, emergency department or inpatient encounter for acute sinusitis, or inpatient encounter for a sinusitis complication, was assessed 1 to 14 days after cohort enrollment. Adverse events were evaluated, including gastrointestinal symptoms, hypersensitivity and skin reactions, acute kidney injury, and secondary infections.
Results: The cohort included 320 141 patients. After propensity score matching, there were 198 942 patients (99 471 patients per group), including 100 340 (50.4%) who were female, 101 726 (51.1%) adolescents aged 12 to 17 years, 52 149 (26.2%) children aged 6 to 11 years, and 45 067 (22.7%) children aged 0 to 5 years. Treatment failure occurred in 1.7% overall; 0.01% had serious failure (an emergency department or inpatient encounter). There was no difference in the risk of treatment failure between the amoxicillin-clavulanate and amoxicillin groups (relative risk [RR], 0.98 [95% CI, 0.92-1.05]). The risk of gastrointestinal symptoms (RR, 1.15 [95% CI, 1.05-1.25]) and yeast infections (RR, 1.33 [95% CI, 1.16-1.54]) was higher with amoxicillin-clavulanate. After patients were stratified by age, the risk of treatment failure after amoxicillin-clavulanate was an RR of 0.98 (95% CI, 0.86-1.12) for ages 0 to 5 years; RR was 1.06 (95% CI, 0.92-1.21) for 6 to 11 years; and RR was 0.87 (95% CI, 0.79-0.95) for 12 to 17 years. The age-stratified risk of adverse events after amoxicillin-clavulanate was an RR of 1.23 (95% CI, 1.10-1.37) for ages 0 to 5 years; RR was 1.19 (95% CI, 1.04-1.35) for 6 to 11 years; and RR was 1.04 (95% CI, 0.95-1.14) for 12 to 17 years.
Conclusions and relevance: In children with acute sinusitis who were treated as outpatients, there was no difference in the risk of treatment failure between those who received amoxicillin-clavulanate compared with amoxicillin, but amoxicillin-clavulanate was associated with a higher risk of gastrointestinal symptoms and yeast infections. These findings may help inform decisions for empirical antibiotic selection in acute sinusitis.
Conflict of interest statement
Figures



Comment in
-
Amoxicillin-Clavulanate vs Amoxicillin for Pediatric Acute Sinusitis.JAMA. 2024 Jan 16;331(3):257-258. doi: 10.1001/jama.2023.23642. JAMA. 2024. PMID: 38227039 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

