Maintenance Therapy With Cetuximab After FOLFIRI Plus Cetuximab for RAS Wild-Type Metastatic Colorectal Cancer: A Phase 2 Randomized Clinical Trial
- PMID: 37721754
- PMCID: PMC10507485
- DOI: 10.1001/jamanetworkopen.2023.33533
Maintenance Therapy With Cetuximab After FOLFIRI Plus Cetuximab for RAS Wild-Type Metastatic Colorectal Cancer: A Phase 2 Randomized Clinical Trial
Abstract
Importance: The optimal maintenance strategy after induction chemotherapy with anti-epidermal growth factor receptor antibody for patients with RAS wild-type metastatic colorectal cancer (mCRC) remains to be debated.
Objective: To evaluate the efficacy and safety of maintenance therapy with single-agent cetuximab after FOLFIRI (leucovorin [folinic acid], fluorouracil, and irinotecan) plus cetuximab induction therapy.
Design, setting, and participants: The TIME (Treatment After Irinotecan-Based Frontline Therapy: Maintenance With Erbitux]) (PRODIGE 28 [Partenariat de Recherche en Oncologie Digestive]-UCGI 27 [UniCancer GastroIntestinal Group]) phase 2 noncomparative, multicenter randomized clinical trial was conducted from January 15, 2014, to November 23, 2018, among 139 patients with unresectable RAS wild-type mCRC. The cutoff date for analysis was July 21, 2022.
Interventions: After first-line induction therapy with 8 cycles of FOLFIRI plus cetuximab, patients without disease progression were randomized (1:1) to biweekly maintenance with cetuximab or observation. On disease progression, the same induction regimen was recommended for 16 weeks followed by further maintenance with cetuximab or observation until disease progression under the full induction regimen.
Main outcomes and measures: The primary end point was the 6-month progression-free rate from randomization. Analysis was performed on an intention-to-treat basis. An exploratory biomolecular analysis, using next-generation sequencing, investigated the putative prognostic value of the tumor mutation profile.
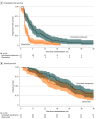
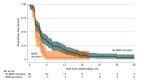
Results: Of 214 patients enrolled (141 men [65.9%]; median age, 67 years [range, 23-85 years]), 139 were randomized to receive cetuximab (n = 67; 45 men [67.2%]; median age, 64 years [range, 34-85 years]) or to be observed (n = 72; 50 men [69.4%]; median age, 68 years [23-85 years]). The 6-month progression-free rate was 38.8% ([26 of 67] 95% CI, 27.1%-51.5%) in the cetuximab group and 5.6% ([4 of 72] 95% CI, 1.5%-13.6%) in the observation group. At a median follow-up of 40.5 months (95% CI, 33.6-47.5 months), median progression-free survival (PFS) from randomization was 5.3 months (95% CI, 3.7-7.4 months) in the cetuximab group and 2.0 months (95% CI, 1.8-2.7 months) in the observation group. Median overall survival (OS) was 24.8 months (95% CI, 18.7-30.4 months) in the cetuximab group and 19.7 months (95% CI, 13.3-24.4 months) in the observation group. In an exploratory multivariate analysis, any tumor-activating mutation in the mitogen-activated protein kinase (MAPK) pathway genes was associated with shorter PFS from randomization regardless of treatment group (hazard ratio, 1.63 [95% CI, 1.01-2.62]; P = .04). The most frequent grade 3 or 4 treatment-related toxic effect in the cetuximab group during maintenance therapy was rash (8 of 67 [11.9%]).
Conclusion and relevance: The randomized clinical trial did not meet its primary end point but suggests clinically meaningful PFS and OS benefits associated with cetuximab maintenance therapy. However, maintenance cetuximab or treatment breaks after first-line combination FOLFIRI-cetuximab therapy seems inappropriate for patients with MAPK-mutated independently of the side of primary tumor. A more complete assessment of MAPK pathway mutations warrants further investigation to the refine treatment strategy for patients with RAS wild-type mCRC.
Trial registration: ClinicalTrials.gov Identifier: NCT02404935.
Conflict of interest statement
Figures
Comment in
-
Anti-Epidermal Growth Factor Receptor Maintenance Therapy in Metastatic Colorectal Cancer-Another Piece to the Puzzle.JAMA Netw Open. 2023 Sep 5;6(9):e2333488. doi: 10.1001/jamanetworkopen.2023.33488. JAMA Netw Open. 2023. PMID: 37721757 No abstract available.
References
-
- Aparicio T, Ghiringhelli F, Boige V, et al. ; PRODIGE 9 Investigators . Bevacizumab maintenance versus no maintenance during chemotherapy-free intervals in metastatic colorectal cancer: a randomized phase III trial (PRODIGE 9). J Clin Oncol. 2018;36(7):674-681. doi:10.1200/JCO.2017.75.2931 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

