Enzymatic Resolution and Decarboxylative Functionalization of α-Sulfinyl Esters
- PMID: 37721804
- PMCID: PMC10872298
- DOI: 10.1002/chem.202302996
Enzymatic Resolution and Decarboxylative Functionalization of α-Sulfinyl Esters
Abstract
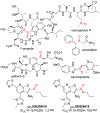
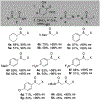
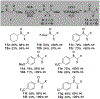
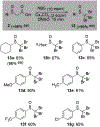
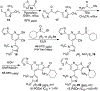
α-Sulfinyl esters can be readily prepared through thiol substitution of α-bromo esters followed by oxidation to the sulfoxide. Enzymatic resolution with lipoprotein lipase provides both the unreacted esters and corresponding α-sulfinyl carboxylic acids in high yields and enantiomeric ratios. Subsequent decarboxylative halogenation, dihalogenation, trihalogenation and cross-coupling gives rise to functionalized sulfoxides. The method has been applied to the asymmetric synthesis of a potent inhibitor of 15-prostaglandin dehydrogenase.
Keywords: decarboxylation; halogenation; lipase; resolution; sulfoxides.
© 2023 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Figures
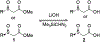
Similar articles
-
Decarboxylative Sulfinylation Enables a Direct, Metal-Free Access to Sulfoxides from Carboxylic Acids.Angew Chem Int Ed Engl. 2022 Oct 24;61(43):e202210525. doi: 10.1002/anie.202210525. Epub 2022 Sep 9. Angew Chem Int Ed Engl. 2022. PMID: 36006859 Free PMC article.
-
Enantiomeric resolution of a series of chiral sulfoxides by high-performance liquid chromatography on polysaccharide-based columns with multimodal elution.J Chromatogr A. 2003 Feb 14;987(1-2):445-52. doi: 10.1016/s0021-9673(02)01451-6. J Chromatogr A. 2003. PMID: 12613840
-
Direct Trideuteromethylation of Sulfenate Anions Generated In Situ from β-Sulfinyl Esters: An Access to Trideuteromethyl Sulfoxides.J Org Chem. 2023 Jul 7;88(13):9505-9513. doi: 10.1021/acs.joc.3c00573. Epub 2023 Jun 7. J Org Chem. 2023. PMID: 37285517
-
Enzymatic Strategies for the Preparation of Pharmaceutically Important Amino Acids through Hydrolysis of Amino Carboxylic Esters and Lactams.Curr Med Chem. 2022;29(41):6218-6227. doi: 10.2174/0929867329666220718123153. Curr Med Chem. 2022. PMID: 35850648 Review.
-
Microbial/enzymatic synthesis of chiral drug intermediates.Adv Appl Microbiol. 2000;47:33-78. doi: 10.1016/s0065-2164(00)47001-2. Adv Appl Microbiol. 2000. PMID: 12876794 Review.
References
-
- Satoh T, Oohara T, Ueda Y, Yamakawa K, J. Org. Chem 1989, 54, 3130–3136;
- Lanfranchi DA, Hanquet G, J. Org. Chem 2006, 71, 4854–4861; - PubMed
- Carmen Carreño M, Hernández-Torres G, Ribagorda M, Urbano A, Chemical Commun. 2009, 6129–6144; - PubMed
- Antczak MI, Cai F, Ready JM, Org. Lett 2011, 13, 184–187; - PMC - PubMed
- Batisse C, Panossian A, Hanquet G, Leroux FR, Chemical Commun. 2018, 54, 10423–10426; - PubMed
- Salom-Roig X, Bauder C, Synthesis 2020, 52, 964–978.
-
- Trost BM, Rao M, Angew. Chem. Int. Ed 2015, 54, 5026–5043; - PubMed
- Angew. Chem 2015, 127, 5112–5130;
- Otocka S, Kwiatkowska M, Madalińska L, Kiełbasiński P, Chem. Rev 2017, 117, 4147–4181; - PubMed
- Jia T, Wang M, Liao J, in Sulfur Chemistry (Ed.: Jiang X), Springer International Publishing, Cham, 2019, pp. 399–427.
-
- Block SS, Stephens RL, Barreto A, Murrill WA, Science 1955, 121, 505–506. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

