Chromophore Planarity, -BH Bridge Effect, and Two-Photon Activity: Bi- and Ter-Phenyl Derivatives as a Case Study
- PMID: 37721870
- PMCID: PMC10544031
- DOI: 10.1021/acs.jpca.3c04288
Chromophore Planarity, -BH Bridge Effect, and Two-Photon Activity: Bi- and Ter-Phenyl Derivatives as a Case Study
Abstract
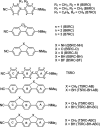
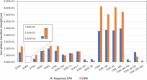
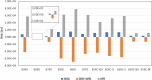
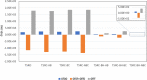
In this work, we have employed electronic structure theories to explore the effect of the planarity of the chromophore on the two-photon absorption properties of bi- and ter-phenyl systems. To that end, we have considered 11 bi- and 7 ter-phenyl-based chromophores presenting a donor-π-acceptor architecture. In some cases, the planarity has been enforced by bridging the rings at ortho-positions by -CH2 and/or -BH, -O, -S, and -NH moieties. The results presented herein demonstrate that in bi- and ter-phenyl systems, the planarity achieved via a -CH2 bridge increases the 2PA activity. However, the introduction of a bridge with the -BH moiety perturbs the electronic structure to a large extent, thus diminishing the two-photon transition strength to the lowest electronic excited state. As far as two-photon absorption activity is concerned, this work hints toward avoiding -BH bridge(s) to enforce planarity in bi- and ter-phenyl systems; however, one may use -CH2 bridge(s) to achieve the enhancement of the property in question. All of these conclusions have been supported by in-depth analyses based on generalized few-state models.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Ma L.-L.; Tang Q.; Liu M.-X.; Liu X.-Y.; Liu J.-Y.; Lu Z.-L.; Gao Y.-G.; Wang R. [12]aneN3-based gemini-type amphiphiles with two-photon absorption properties for enhanced nonviral gene delivery and bioimaging. ACS Appl. Mater. Interfaces 2020, 12, 40094–40107. 10.1021/acsami.0c10718. - DOI - PubMed
-
- Sun C.-L.; Li J.; Wang X.-Z.; Shen R.; Liu S.; Jiang J.-Q.; Li T.; Song Q.-W.; Liao Q.; Fu H.-B.; Yao J.-N.; Zhang H.-L. Rational design of organic probes for turn-on two-photon excited fluorescence imaging and photodynamic therapy. Chem 2019, 5, 600–616. 10.1016/j.chempr.2018.12.001. - DOI
-
- Yang L.-L.; Zhang L.; Wan S.-C.; Wang S.; Wu Z.-Z.; Yang Q.-C.; Xiao Y.; Deng H.; Sun Z.-J. Two-photon absorption induced cancer immunotherapy using covalent organic frameworks. Adv. Funct. Mater. 2021, 31, 2103056 10.1002/adfm.202103056. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

