Gardnerella Vaginolysin Potentiates Glycan Molecular Mimicry by Neisseria gonorrhoeae
- PMID: 37722688
- PMCID: PMC10681867
- DOI: 10.1093/infdis/jiad391
Gardnerella Vaginolysin Potentiates Glycan Molecular Mimicry by Neisseria gonorrhoeae
Abstract
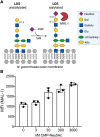
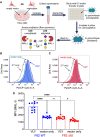
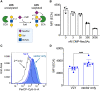
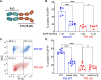
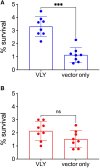
Bacterial vaginosis (BV) is a dysbiotic condition of the vaginal microbiome associated with higher risk of infection by Neisseria gonorrhoeae-the cause of gonorrhea. Here we test if one known facet of BV-the presence of bacterial cytolysins-leads to mobilization of intracellular contents that enhance gonococcal virulence. We cloned and expressed recombinant vaginolysin (VLY), a cytolysin produced by the BV-associated bacterium Gardnerella, verifying that it liberates contents of cervical epithelial (HeLa) cells, while vector control preparations did not. We tested if VLY mediates a well-known gonococcal virulence mechanism-the molecular mimicry of host glycans. To evade host immunity, N. gonorrhoeae caps its lipooligosaccharide (LOS) with α2-3-linked sialic acid. For this, gonococci must scavenge a metabolite made inside host cells. Flow cytometry-based lectin-binding assays showed that gonococci exposed to vaginolysin-liberated contents of HeLa cells displayed greater sialic acid capping of their LOS. This higher level of bacterial sialylation was accompanied by increased binding of the complement regulatory protein factor H, and greater resistance to complement attack. Together these results suggest that cytolytic activities present during BV may enhance the ability of N. gonorrhoeae to capture intracellular metabolites and evade host immunity via glycan molecular mimicry.
Keywords: Gardnerella; bacterial vaginosis; complement; cytolysin; factor H; gonorrhea; sialic acid; vaginolysin.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. S. R. is a cofounder of STIRx, Inc. All other authors declare no conflict of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


Similar articles
-
α-2,3-sialyltransferase expression level impacts the kinetics of lipooligosaccharide sialylation, complement resistance, and the ability of Neisseria gonorrhoeae to colonize the murine genital tract.mBio. 2015 Feb 3;6(1):e02465-14. doi: 10.1128/mBio.02465-14. mBio. 2015. PMID: 25650401 Free PMC article.
-
Neisseria gonorrhoeae scavenges host sialic acid for Siglec-mediated, complement-independent suppression of neutrophil activation.mBio. 2024 May 8;15(5):e0011924. doi: 10.1128/mbio.00119-24. Epub 2024 Apr 9. mBio. 2024. PMID: 38587424 Free PMC article.
-
Cholesterol-Dependent Cytolysins Produced by Vaginal Bacteria: Certainties and Controversies.Front Cell Infect Microbiol. 2020 Jan 10;9:452. doi: 10.3389/fcimb.2019.00452. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 31998661 Free PMC article. Review.
-
The Lst Sialyltransferase of Neisseria gonorrhoeae Can Transfer Keto-Deoxyoctanoate as the Terminal Sugar of Lipooligosaccharide: a Glyco-Achilles Heel That Provides a New Strategy for Vaccines to Prevent Gonorrhea.mBio. 2021 Mar 23;12(2):e03666-20. doi: 10.1128/mBio.03666-20. mBio. 2021. PMID: 33758087 Free PMC article.
-
Species-specificity of Neisseria gonorrhoeae infection: do human complement regulators contribute?Vaccine. 2008 Dec 30;26 Suppl 8:I62-6. doi: 10.1016/j.vaccine.2008.11.051. Vaccine. 2008. PMID: 19388167 Review.
Cited by
-
Social, microbial, and immune factors linking bacterial vaginosis and infectious diseases.J Clin Invest. 2025 Jun 2;135(11):e184322. doi: 10.1172/JCI184322. eCollection 2025 Jun 2. J Clin Invest. 2025. PMID: 40454473 Free PMC article. Review.
-
Identification and characterisation of vaginal bacteria-glycan interactions implicated in reproductive tract health and pregnancy outcomes.Nat Commun. 2025 Jun 5;16(1):5207. doi: 10.1038/s41467-025-60404-1. Nat Commun. 2025. PMID: 40467588 Free PMC article.
-
Bacterial vaginosis.Nat Rev Dis Primers. 2025 Jun 19;11(1):43. doi: 10.1038/s41572-025-00626-1. Nat Rev Dis Primers. 2025. PMID: 40537474 Review.
-
The role of sialidases in the pathogenesis of bacterial vaginosis and their use as a promising pharmacological target in bacterial vaginosis.Front Cell Infect Microbiol. 2024 Mar 1;14:1367233. doi: 10.3389/fcimb.2024.1367233. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38495652 Free PMC article. Review.
References
-
- Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 2007; 34:864–9. - PubMed
-
- Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 74:14–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

