Severe Cytokine Release Syndrome and Immune Effector Cell-associated Neurotoxicity Syndrome in a Man Receiving Immune Checkpoint Inhibitors for Lung Cancer
- PMID: 37722894
- PMCID: PMC11116002
- DOI: 10.2169/internalmedicine.2429-23
Severe Cytokine Release Syndrome and Immune Effector Cell-associated Neurotoxicity Syndrome in a Man Receiving Immune Checkpoint Inhibitors for Lung Cancer
Abstract
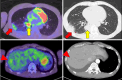
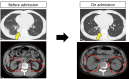
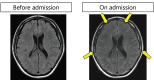
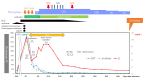
A 55-year-old man with stage IV lung adenocarcinoma was treated with cisplatin, pemetrexed, nivolumab, and ipilimumab. Approximately 100 days after treatment initiation, he became disoriented and presented to the emergency department with a high fever. Blood tests revealed liver and kidney dysfunctions. Subsequently, the patient developed generalized convulsions that required intensive care. He was clinically diagnosed with cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Organ damage was gradually controlled with immunosuppressive drugs, including steroids, and the patient was discharged. Successful treatment is rare in patients with CRS, including ICANS, during immune checkpoint inhibitor treatment for solid tumors.
Keywords: cytokine release syndrome; immune checkpoint inhibitor; immune effector cell-associated neurotoxicity syndrome; ipilimumab; nivolumab.
Conflict of interest statement
Figures
References
-
- Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol 39: 3978-3992, 2021. - PubMed
-
- de Groot PM, Arevalo O, Shah K, et al. Imaging primer on chimeric antigen receptor T-cell therapy for radiologists. Radiographics 42: 176-194, 2022. - PubMed

