DOPA decarboxylase is an emerging biomarker for Parkinsonian disorders including preclinical Lewy body disease
- PMID: 37723208
- PMCID: PMC10570139
- DOI: 10.1038/s43587-023-00478-y
DOPA decarboxylase is an emerging biomarker for Parkinsonian disorders including preclinical Lewy body disease
Abstract
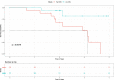
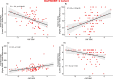
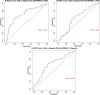
The diagnosis of Parkinsonian disorders is currently based on clinical criteria, which have limited sensitivity until most dopaminergic neurons are lost. Here we show that cerebrospinal fluid levels of DOPA decarboxylase (DDC) (also known as aromatic L-amino acid decarboxylase) can accurately identify patients with Lewy body disease (LBD) (area under the curve (AUC) = 0.89; PFDR = 2.6 × 10-13) and are associated with worse cognitive performance (P < 0.05). We also found that DDC can detect preclinical LBD stages in clinically unimpaired individuals with a positive seed amplification α-synuclein assay (AUC = 0.81, P = 1.0 × 10-5) and that this biomarker could predict progression to clinical LBD over a 3-year period in preclinical cases (hazard ratio = 3.7 per s.d. change, confidence interval = 1.1-12.7). Moreover, DDC levels were also increased in atypical Parkinsonian disorders but not in non-Parkinsonian neurodegenerative disorders. These cerebrospinal fluid results were replicated in an independent cohort, where we also found that DDC levels in plasma could identify both LBD and atypical Parkinsonian disorders (AUC = 0.92, P = 1.3 × 10-14). Our results show that DDC might have a future role in clinical practice as a biomarker of dopaminergic dysfunction to detect Parkinsonian disorders even during the preclinical disease stages and predict their progression to clinical LBD.
© 2023. The Author(s).
Conflict of interest statement
O.H. has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer and Roche. In the past 2 years, he has received consultancy and speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Genentech, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. S.P. has acquired research support (for the institution) from ki elements/ADDF. In the past 2 years, he has received consultancy and speaker fees from Bioartic, Biogen, Cytox, Eli Lilly, Geras Solutions and Roche. The other authors declare no competing interests.
Figures








Comment in
-
An emerging biomarker for dopaminergic cell loss.Nat Aging. 2023 Oct;3(10):1180-1182. doi: 10.1038/s43587-023-00501-2. Nat Aging. 2023. PMID: 37735241 No abstract available.
-
Tracking neurodegenerative diseases in biofluids: Combining pathology and pathophysiology markers is the way.Cell Chem Biol. 2023 Nov 16;30(11):1340-1342. doi: 10.1016/j.chembiol.2023.10.018. Cell Chem Biol. 2023. PMID: 37977130
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

