The clinical utility and costs of whole-genome sequencing to detect cancer susceptibility variants-a multi-site prospective cohort study
- PMID: 37723522
- PMCID: PMC10507925
- DOI: 10.1186/s13073-023-01223-1
The clinical utility and costs of whole-genome sequencing to detect cancer susceptibility variants-a multi-site prospective cohort study
Abstract
Background: Many families and individuals do not meet criteria for a known hereditary cancer syndrome but display unusual clusters of cancers. These families may carry pathogenic variants in cancer predisposition genes and be at higher risk for developing cancer.
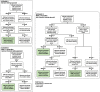
Methods: This multi-centre prospective study recruited 195 cancer-affected participants suspected to have a hereditary cancer syndrome for whom previous clinical targeted genetic testing was either not informative or not available. To identify pathogenic disease-causing variants explaining participant presentation, germline whole-genome sequencing (WGS) and a comprehensive cancer virtual gene panel analysis were undertaken.
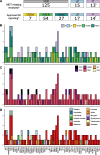
Results: Pathogenic variants consistent with the presenting cancer(s) were identified in 5.1% (10/195) of participants and pathogenic variants considered secondary findings with potential risk management implications were identified in another 9.7% (19/195) of participants. Health economic analysis estimated the marginal cost per case with an actionable variant was significantly lower for upfront WGS with virtual panel ($8744AUD) compared to standard testing followed by WGS ($24,894AUD). Financial analysis suggests that national adoption of diagnostic WGS testing would require a ninefold increase in government annual expenditure compared to conventional testing.
Conclusions: These findings make a case for replacing conventional testing with WGS to deliver clinically important benefits for cancer patients and families. The uptake of such an approach will depend on the perspectives of different payers on affordability.
Keywords: Diagnostic testing; Familial cancer; Genetics; Health economics; Variants; Whole-genome sequencing.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
J.V.P and N.W are founders of genomiQa Pty Ltd, and members of its Board. G.E.H is the clinical genomics lead at genomiQa. R.L.W and S.N are members of the Medical Services Advisory Committee (MSAC) which provides advice to the Australian Government on public reimbursement of services and tests (which includes genomics). The remaining authors declare that they have no competing interests.
Figures

References
-
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

