Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study
- PMID: 37723577
- PMCID: PMC10506267
- DOI: 10.1186/s13075-023-03128-1
Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study
Abstract
Background: Upadacitinib, a Janus kinase inhibitor, has demonstrated efficacy and an acceptable safety profile in patients with ankylosing spondylitis (AS) in the phase III SELECT-AXIS programs. We report the 1-year efficacy and safety in patients with AS and an inadequate response to biologic disease-modifying antirheumatic drugs (bDMARD-IR) from the SELECT-AXIS 2 study.
Methods: Patients ≥ 18 years with active AS who met the modified New York criteria for AS and were bDMARD-IR received double-blind upadacitinib 15 mg once daily (QD) or placebo for 14 weeks. Patients who completed 14 weeks could enter an open-label extension and receive upadacitinib 15 mg QD for up to 2 years. Efficacy endpoints included the percentage of patients achieving ≥ 40% improvement in Assessment of SpondyloArthritis international Society response (ASAS40), Ankylosing Spondylitis Disease Activity Score (ASDAS) low disease activity (LDA), and ASDAS inactive disease (ID); and change from baseline in total and nocturnal back pain, and Bath Ankylosing Spondylitis Functional Index (BASFI). Subgroup analyses (bDMARD lack of efficacy versus intolerance, and prior tumor necrosis factor inhibitor [TNFi] versus interleukin-17 inhibitor [IL-17i] exposure) were conducted. Binary and continuous efficacy endpoints were assessed using non-responder imputation with multiple imputation (NRI-MI) and as observed (AO) analyses; and mixed-effects model repeated measures (MMRM) and AO, respectively. Safety was assessed based on adverse events. Data through week 52 are reported.
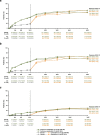
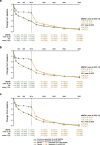
Results: Of 420 randomized patients, 366 (continuous upadacitinib: n = 181; placebo to upadacitinib: n = 185) completed 52 weeks of treatment. At week 52, in the continuous upadacitinib and placebo to upadacitinib groups, ASAS40, ASDAS LDA, and ASDAS ID were achieved by 66% and 65%, 57% and 55%, and 26% and 25% (all NRI-MI); and change from baseline in total back pain, nocturnal back pain, and BASFI was -4.5 and -4.3, -4.6 and -4.4, and -3.6 and -3.5 (all MMRM), respectively. No new safety risks were identified. Subgroup analyses were consistent with the overall study population.
Conclusions: Upadacitinib 15 mg QD demonstrated sustained improvement up to 52 weeks in bDMARD-IR patients with AS. Efficacy was generally similar in patients with lack of efficacy versus intolerance to bDMARDs and prior TNFi versus IL-17i exposure.
Trial registration: NCT02049138.
Keywords: Ankylosing spondylitis; Biologic; Inadequate response; Janus kinase inhibitor; Open-label extension; Refractory; Tumor necrosis factor; Upadacitinib.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
XB has received grant/research support from AbbVie and Novartis; consulting fees from AbbVie, BMS, Chugai, MSD, Novartis, Pfizer, and UCB; speakers’ bureau fees from AbbVie, BMS, Celgene, Chugai, Merck, Novartis, Pfizer, and UCB; and is an editorial board member of the
Figures

References
-
- de Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther. 2016;18(1):196. doi: 10.1186/s13075-016-1093-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

